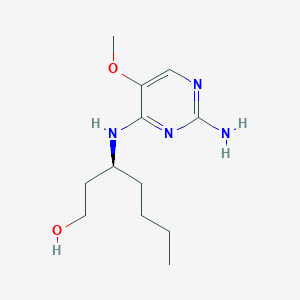
(3S)-3-[(2-amino-5-methoxypyrimidin-4-yl)amino]heptan-1-ol
for the treatment of viral infections
| Molecular Formula: | C12H22N4O2 |
|---|---|
| Molecular Weight: | 254.32868 g/mol |
To the use of pyrimidine derivatives in the treatment of viral infections, immune or inflammatory disorders, whereby the modulation, or agonism, of toll-like-receptors (TLRs) is involved. Toll-Like Receptors are primary transmembrane proteins characterized by an extracellular leucine rich domain and a cytoplasmic extension that contains a conserved region. The innate immune system can recognize pathogen-associated molecular patterns via these TLRs expressed on the cell surface of certain types of immune cells. Recognition of foreign pathogens activates the production of cytokines and upregulation of co-stimulatory molecules on phagocytes. This leads to the modulation of T cell behaviour.
It has been estimated that most mammalian species have between ten and fifteen types of Toll-like receptors. Thirteen TLRs (named TLR1 to TLR13) have been identified in humans and mice together, and equivalent forms of many of these have been found in other mammalian species. However, equivalents of certain TLR found in humans are not present in all mammals. For example, a gene coding for a protein analogous to TLR10 in humans is present in mice, but appears to have been damaged at some point in the past by a retrovirus. On the other hand, mice express TLRs 11, 12, and 13, none of which are represented in humans. Other mammals may express TLRs which are not found in humans. Other non-mammalian species may have TLRs distinct from mammals, as demonstrated by TLR14, which is found in the Takifugu pufferfish. This may complicate the process of using experimental animals as models of human innate immunity.
For detailed reviews on toll-like receptors see the following journal articles. Hoffmann, J. A., Nature, 426, p 33-38, 2003; Akira, S., Takeda, K., and Kaisho, T., Annual Rev. Immunology, 21, p 335-376, 2003; Ulevitch, R. J., Nature Reviews: Immunology, 4, p 512-520, 2004.
Compounds indicating activity on Toll-Like receptors have been previously described such as purine derivatives in WO 2006/117670, adenine derivatives in WO 98/01448 and WO 99/28321, and pyrimidines in WO 2009/067081. However, there exists a strong need for novel Toll-Like receptor modulators having preferred selectivity, higher potency, higher metabolic stability, and an improved safety profile compared to the compounds of the prior art.
In the treatment of certain viral infections, regular injections of interferon (IFNα) can be administered, as is the case for hepatitis C virus (HCV), (Fried et. al. Peginterferon-alfa plus ribavirin for chronic hepatitis C virus infection, N Engl J Med 2002; 347: 975-82). Orally available small molecule IFN inducers offer the potential advantages of reduced immunogenicity and convenience of administration. Thus, novel IFN inducers are potentially effective new class of drugs for treating virus infections. For an example in the literature of a small molecule IFN inducer having antiviral effect see De Clercq, E.; Descamps, J.; De Somer, P. Science 1978, 200, 563-565.
IFNα is also given in combination with other drugs in the treatment of certain types of cancer (Eur. J. Cancer 46, 2849-57, and Cancer Res. 1992, 52, 1056). TLR 7/8 agonists are also of interest as vaccine adjuvants because of their ability to induce pronounced Th1 response (Hum. Vaccines 2010, 6, 1-14; Hum. Vaccines 2009, 5, 381-394).
50d
Paper

Toll-like receptor (TLR) 7 and 8 agonists can potentially be used in the treatment of viral infections and are particularly promising for chronic hepatitis B virus (HBV) infection. An internal screening effort identified a pyrimidine Toll-like receptor 7 and 8 dual agonist. This provided a novel alternative over the previously reported adenine and pteridone type of agonists. Structure–activity relationship, lead optimization, in silico docking, pharmacokinetics, and demonstration of ex vivo and in vivo cytokine production of the lead compound are presented.
Novel Pyrimidine Toll-like Receptor 7 and 8 Dual Agonists to Treat Hepatitis B Virus
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015274676 | 2015-10-01 | ACYLAMINOPYRIMIDINE DERIVATIVES FOR THE TREATMENT OF VIRAL INFECTIONS AND FURTHER DISEASES |
| US2014045849 | 2014-02-13 | PYRIMIDINE DERIVATIVES FOR THE TREATMENT OF VIRAL INFECTIONS |
/////////////// treatment of viral infections, Pyrimidine Toll-like Receptor 7, Toll-like Receptor 8 Dual Agonists, Treat Hepatitis B Virus, PRECLINICAL
n1c(nc(c(c1)OC)N[C@@H](CCCC)CCO)N
Filed under: Preclinical drugs Tagged: preclinical, Pyrimidine Toll-like Receptor 7, Toll-like Receptor 8 Dual Agonists, Treat Hepatitis B Virus, treatment of viral infections