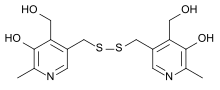

Pyritinol
- Molecular FormulaC16H20N2O4S2
- Average mass368.471 Da
1098-97-1[RN]
1308
214-150-1[EINECS]
233-178-5[EINECS]
3,3′-[Dithiobis(methylene)]bis[5-hydroxy-6-methyl-4-pyridinemethanol]
4-Pyridinemethanol, 3,3′-[dithiobis(methylene)]bis[5-hydroxy-6-methyl-
пиритинол[Russian][INN]
بيريتينول[Arabic][INN]
吡硫醇[Chinese][INN]
Pyritinol, CAS Registry Number: 1098-97-1
CAS Name: 3,3¢-[Dithiobis(methylene)]bis[5-hydroxy-6-methyl-4-pyridinemethanol]
Additional Names: bis(4-hydroxymethyl-5-hydroxy-6-methyl-3-pyridylmethyl) disulfide; bis[(3-hydroxy-4-hydroxymethyl-2-methyl-5-pyridyl)methyl] disulfide; dipyridoxolyldisulfide; pyridoxine-5-disulfide; pyrithioxin
Molecular Formula: C16H20N2O4S2, Molecular Weight: 368.47
Percent Composition: C 52.15%, H 5.47%, N 7.60%, O 17.37%, S 17.40%
Literature References: Prepn: Zima, Schorre, US3010966 (1961 to E. Merck); Iwanami et al.,Bitamin36, 122 (1967); J. Vitaminol.14, 321, 326 (1968). HPLC determn in urine: K. Kitao et al.,Chem. Pharm. Bull.25, 1335 (1977). Pharmacokinetics and metabolism: Darge et al.,Arzneim.-Forsch.19, 5, 9, (1969); Nowak, Schorre, ibid. 11. Clinical trial in dementia: S. Hoyer et al.,ibid.27, 671 (1977); A. J. Cooper, R. V. Magnus, Pharmacotherapeutica2, 317 (1980); in cerebrovascular disorders: Y. Tazaki et al.,J. Int. Med. Res.8, 118 (1980).
Properties: Crystals, mp 218-220°.
Melting point: mp 218-220°
Derivative Type: Dihydrochloride monohydrate
Trademarks: Biocefalin (Benvegna); Bonifen (Merck KGaA); Enbol (Chugai); Encephabol (Merck KGaA); Enerbol (Polfa); Epocan (Merck KGaA); Life (SIT)
Molecular Formula: C16H20N2O4S2.2HCl.H2O, Molecular Weight: 459.41
Percent Composition: C 41.83%, H 5.27%, N 6.10%, O 17.41%, S 13.96%, Cl 15.43%
Properties: mp 184°. Note: Has no vitamin B6 activity.
Melting point: mp 184°
Therap-Cat: Nootropic.
Keywords: Nootropic.
Derivatives
Dihydrochloride monohydrate
- Formula:C16H20N2O4S2 • 2HCl • H2O
- MW:459.42 g/mol
- CAS-RN:10049-83-9
- EINECS:233-178-5
- LD50:221 mg/kg (M, i.v.); 5786 mg/kg (M, p.o.);
300 mg/kg (R, i.v.); 6 g/kg (R, p.o.)
Pyritinol has been used in trials studying the treatment of Dementia, Depression, Schizophrenia, Anxiety Disorders, and Psychosomatic Disorders.
Pyritinol also called pyridoxine disulfide or pyrithioxine (European drug names Encephabol, Encefabol, Cerbon 6) is a semi-synthetic water-soluble analog of vitamin B6 (Pyridoxine HCl). It was produced in 1961 by Merck Laboratories by bonding 2 vitamin B6 compounds (pyridoxine) together with a disulfide bridge. Since the 1970s, it has been a prescription and OTC drug in several countries for cognitive disorders, rheumatoid arthritis,[1] and learning disorders in children. Since the early 1990s it has been sold as a nootropic dietary supplement in the United States.

SYN
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 39984-49-1 | C8H10Br3NO | 3,4-bis(bromomethyl)-5-hydroxy-6-methylpyridine hydrobromide | 3-Pyridinol, 4,5-bis(bromomethyl)-2-methyl- |
| 92147-37-0 | C11H15NO3S2 | ethylxanthic acid [5-hydroxy-4-(hydroxymethyl)-6-methyl-3-pyridyl]methyl ester | Xanthic acid, ethyl-, [5-hydroxy-4-(hydroxymethyl)-6-methyl-3-pyridyl]methyl ester |
| 140-89-6 | C3H5KOS2 | potassium ethylxanthogenate | Carbonodithioic acid, O-ethyl ester, potassium salt |

PATENT
PATENT
https://patents.google.com/patent/CN103992268A/en
Pyritinol, it is the derivative of vitamin B6, for nootropic agents, can promote glucose and amino acid metabolism in brain, improve whole body assimilation, increase Flow of carotid artery, improve cerebral blood flow (CBF), be applicable to the dizzy distending pain, insomnia, hypomnesis of cerebral trauma sequela, encephalitis and meningitis sequela etc., the improvement of absent minded, emotional change; Also for cerebral arteriosclerosis, senile dementia mental symptom etc.
The pyritinol of applying clinically at present, it is pyritinol hydrochloride, be specially the monohydrate of hydrochloride, its chemical name is 3,3-(dithio methylene radical) two (5-hydroxyl-6-methyl-pyridine methane) dihydrochloride monohydrate, has recorded in < < Chinese Pharmacopoeia version > > in 2010.The preparation of this product listing has sheet, capsule and sterile powder injection, and its injection easily causes venous stimulation when clinical application, has greatly limited clinical application.The powder injection of pyritinol hydrochloride easy caking after standing storage, not soluble or dissolve and thoroughly cause liquid unclarity, particulate matter to exceed standard and easily cause the untoward reactions such as Microembolization during use.
CN101003509A discloses hydrobromate and the mesylate of pyritinol, record its stability having had, solvability and bland advantage, but in fact, Hydrogen bromide pyritinol, methylsulfonic acid pyritinol store easy moisture absorption under normal condition, in purification refine, be difficult to separate out with conventional crystallization method, need loaded down with trivial details aftertreatment technology, Hydrogen bromide and methylsulfonic acid have strong corrodibility in addition, comparatively difficult to its suitability for industrialized production.
CN101066266A discloses organic acid salt of pyritinol and preparation method thereof, wherein preferred pyritinol nicotinate.Yet, in nicotinic acid pyritinol water solvability a little less than, and nicotinic acid pyritinol preparation technology used dry-out benzene, toxicity is larger, and aftertreatment technology is complicated, is not suitable for suitability for industrialized production.
Yet, existing pyritinol or its salt, or pyritinol salt exists defect in the use, or the production technique that obtains this pyritinol salt is unsuitable for suitability for industrialized production.For this reason, need to provide a kind of safe, pyritinol salt and production method thereof of stablizing, meeting industrialization production requirements.
Embodiment 1: pyritinol maleate synthetic
Get 5.0g pyritinol powder, drop in reaction flask, add 100ml purified water, then under agitation add toxilic acid 3.8g, finish, be heated to 60-65 ℃ and stir 30min and all dissolve to solid, remove heating fluid, stirred crystallization under room temperature, separate out a large amount of white solids, use a small amount of cold water washing, 45 ℃ of vacuum-dryings, obtain white powder 5.97g, yield 72.9%.Purity: 99.5%; M.p.:134~137 ℃; Ultimate analysis (C16H20N2O4S22C4H4O4): C:47.9%, H:4.8%, N:4.6%, S:10.6%, O:32.1% (theory: C:48.0%, H:4.7%, N:4.7%, S:10.7%, O:32.0%); 1H-NMR (600MHz, DMSO) δ: 2.39 (6H, s), 3.93 (4H, s), 4.76 (4H, s), 6.18 (4H, s), 7.87 (2H, s).By the 1H-NMR (Fig. 2) of toxilic acid pyritinol and the 1H-NMR (Fig. 1) of pyritinol contrast, in a part toxilic acid pyritinol, contain 2 molecule toxilic acids.
Embodiment 2: pyritinol maleate synthetic
Get 5.0g pyritinol powder, drop in reaction flask, add 100ml ethanol, then under agitation add toxilic acid 3.0g, finish, be heated to return stirring 30min and all dissolve to solid, remove heating fluid, stirred crystallization under room temperature, separate out a large amount of white solids, use a small amount of cold water washing, 45 ℃ of vacuum-dryings, obtain white powder 5.50g, yield 67.5%.After measured, the toxilic acid pyritinol that structure makes with embodiment 1.
PATENT
https://patents.google.com/patent/CN105153021A/en
Embodiment 1
Toxilic acid 3.8g is dissolved in 100ml ethanol, be warming up to 60 DEG C clearly molten, add pyritinol 5.0g, stir clearly molten, react 1 hour, cooling crystallization, filter, solid is drying under reduced pressure at 50 DEG C, obtains white crystalline solid toxilic acid pyritinol crystal form A 4.9g.X-ray powder diffraction analysis, as Fig. 1, its 2 θ value is as following table.
Embodiment 2
Toxilic acid 3.8g is dissolved in 100ml acetone, be warming up to 45 DEG C clearly molten, add pyritinol 5.0g, stir clearly molten, react 1.5 hours, cooling crystallization, filter, solid is drying under reduced pressure at 50 DEG C, obtains white crystalline solid 5.2g.It is toxilic acid pyritinol crystal form A that dry product does X-ray powder diffraction.
Embodiment 3
Toxilic acid 3.8g is dissolved in and adds 100ml Virahol, be warming up to 60 DEG C clearly molten, add pyritinol 5.0g, stir clearly molten, react 2 hours, cooling crystallization, filter, solid is drying under reduced pressure at 50 DEG C, obtains white crystalline solid 5.1g.It is toxilic acid pyritinol crystal form A that dry product does X-ray powder diffraction.
PATENT
https://patents.google.com/patent/CN101066266A/en
Specific embodiment:
Embodiment 1: nicotinic acid pyritinol salt synthetic
Get nicotinic acid 24.6g, fully be dissolved in the 300ml anhydrous benzene, heated and stirred is to molten entirely, under complete molten state, add pyritinol 40.5g, reflux mixture 3 hours, TLC thin layer identification (developing solvent: ethyl acetate: ethanol: glacial acetic acid=5: 6: 0.6) fully, the cooling back adds the 200ml dehydrated alcohol slightly, mixture is put into refrigerator fully cool off, sucking filtration is separated out white crystals, with a small amount of cold absolute ether washing solid.65 ℃ of vacuum dryings get 62.1g nicotinic acid pyritinol salt, yield 89.7%.Determination of acid-basetitration nicotinic acid and pyritinol content are measured moisture with the karl Fischer method.The result is: nicotinic acid 37.2%, and pyritinol 62.0%, water 5.8%, approaching with theoretical value, contain 2 water of crystallization.Elementary analysis: theoretical value C52.8% H5.3% O25.2%N6.6% S10.1%; Measured value C52.4% H5.2% O25.1%N6.5% S10.0%.
Embodiment 2: fumaric acid pyritinol salt synthetic
Get fumaric acid 11.6g, fully be dissolved in the 300ml anhydrous benzene, heated and stirred is to molten entirely, under complete molten state, add pyritinol 40.5g, reflux mixture 3 hours, TLC thin layer identification (developing solvent: ethyl acetate: ethanol: glacial acetic acid=5: 4: 0.8) fully, the cooling back adds the 200ml dehydrated alcohol slightly, mixture is put into refrigerator fully cool off, sucking filtration is separated out white crystals, with a small amount of cold absolute ether washing solid.65 ℃ of vacuum dryings get 49.9g fumaric acid pyritinol salt, yield 88.9%.Determination of acid-basetitration fumaric acid and pyritinol content are measured moisture with the karl Fischer method.The result is: fumaric acid 20.8%, and pyritinol 72.7%, water 6.5%, approaching with theoretical value, contain 2 water of crystallization.Elementary analysis: theoretical value C49.6% H5.0%O26.4% N5.8% S13.2%; Measured value C49.4% H5.2% O26.5% N5.9%S13.1%.
PATENT
https://patents.google.com/patent/CN102516297A/en
Embodiment 1: the preparation of compd A
With Pyrithioxine hydrochloride 10g, be dissolved in the 20ml pyridine, slowly drip POCl3 solution 10ml under the room temperature; Drip and finish, stirring at room reaction 12 hours slowly adds the 100g frozen water and stirred hydrolysis reaction 2 hours; Toluene gradation extraction 30ml * 3, water layer evaporated under reduced pressure, Virahol dissolution residual substance; Filter, evaporate to dryness gets compd A 4.2g.
Embodiment 2: the preparation of compd B
With Pyrithioxine hydrochloride 10g, be dissolved in the 40ml THF, add 4gNaH, 30 ℃ were stirred 2 hours; Add the 20ml POCl3, stirring reaction 16 hours slowly adds the 100g frozen water and stirred hydrolysis reaction 2 hours; ETHYLE ACETATE gradation extraction 30ml * 3, the water layer evaporated under reduced pressure adds 80ml Virahol dissolution residual substance; Add 40ml water, freezing crystallization gets compd B 5.6g.
Embodiment 3: the preparation of Compound C
With Pyrithioxine hydrochloride 10g, be dissolved in the 40ml THF, add 4gNaH, 30 ℃ were stirred 2 hours; Add the 20ml chloroiodomethane, stirring reaction 16 hours, 60 ℃ of evaporated under reduced pressure add 20ml acetonitrile dissolution residual substance; As midbody, other gets triethylamine 9ml and is dissolved in the 10ml acetonitrile, drips 3.6ml phosphoric acid, after dropping finishes; Stir down and slowly splash into midbody, continued 60 ℃ of stirring reactions 12 hours, steaming desolventizes; Residue adds water 20ml dissolving, and water layer filters clarification, and freeze-drying promptly gets compd B 6.7g.
Embodiment 4: the preparation of Compound D
Serine 3 grams, ethylene bromohyrin 2.5g, N with the BOC protection; N-Dimethylamino pyridine 3g and NSC 57182 3g are dissolved in the THF; Stirring at room 10 hours, vacuum concentration is with the thick product of chromatography purification (with the ETHYLE ACETATE/normal hexane wash-out of normal hexane to 30%); Merging filtrate, evaporate to dryness gets intermediate A; Pyrithioxine hydrochloride 2g and intermediate A 2.5g are dissolved with THF 30ml, add triphenyl phosphorus 2g, slowly drip diethyl azodiformate solution 2ml, room temperature reaction 5 hours; Reaction is finished, and evaporated under reduced pressure adds ETHYLE ACETATE 50ml dissolving, filters insolubles; With the thick product of chromatography purification (with the ETHYLE ACETATE/normal hexane wash-out of normal hexane to 10%), merging filtrate, evaporate to dryness dissolves with methylene dichloride 20ml then; Feed hydrogen chloride gas to saturated, stirring reaction 5 hours filters; Get the hydrochloride of Compound D, transferring pH behind the use dissolved in distilled water is about 8, and the water layer lyophilize gets Compound C 0.27g.
Embodiment 5: the preparation of compd E
Get compd A 10g, be dissolved in the 30ml Virahol, add 25gBoc-Ser-OBZL in batches, 50 ℃ of stirring reactions; HPLC monitoring react to compd B less than 5%, add 0.1M hydrochloric acid soln 20ml, 60 ℃ of heating hydrolysis 5 hours are regulated pH to 7; Evaporated under reduced pressure adds anhydrous alcohol solution, removes by filter insolubles, evaporated under reduced pressure; Add the 5ml water dissolution, filtering, lyophilize get compd E 6.9g
///////////////////////////////////////////
AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Availability
It is approved for “symptomatic treatment of chronically impaired brain function in dementia syndromes” and for “supportive treatment of sequelae of craniocerebral trauma” in various European countries, including Austria, Germany, France, Italy, Portugal, and Greece. In France it is also approved for rheumatoid arthritis as a disease modifying drug, on the basis of the results of clinical trials. In many countries it is available over the counter and is widely advertised on the internet as being for “memory disturbances.”
Effects
review refs needed
Adverse effects
Adverse effects include nausea, headache,[2] and rarely allergic reaction (mild skin reactions).[3] A 2004 survey of six case reports suggested a link between pyritinol and severe cholestatic hepatitis when on several drugs for certain diseases.[4]
Other rare side effects: acute pancreatitis[5] and photoallergic eruption.[6]
References
- ^ Lemmel EM (May 1993). “Comparison of pyritinol and auranofin in the treatment of rheumatoid arthritis. The European Multicentre Study Group”. British Journal of Rheumatology. 32 (5): 375–82. doi:10.1093/rheumatology/32.5.375. PMID 8495257.
- ^ Nachbar F, Korting HC, Vogl T (1993). “Erythema multiforme-like eruption in association with severe headache following pyritinol”. Dermatology. 187 (1): 42–6. doi:10.1159/000247196. PMID 8324277.
- ^ de Groot, Anton C.; Nater, Johan Pieter; Weyland, J. Willem. Unwanted Effects of Cosmetics and Drugs Used in Dermatology.[full citation needed][page needed]
- ^ Maria V, Albuquerque A, Loureiro A, Sousa A, Victorino R (March 2004). “Severe cholestatic hepatitis induced by pyritinol”. BMJ. 328 (7439): 572–4. doi:10.1136/bmj.328.7439.572. PMC 381054. PMID 15001508.
- ^ Straumann A, Bauer M, Pichler WJ, Pirovino M (August 1998). “Acute pancreatitis due to pyritinol: an immune-mediated phenomenon”. Gastroenterology. 115 (2): 452–4. doi:10.1016/S0016-5085(98)70212-4. PMID 9679051.
- ^ Tanaka M, Niizeki H, Shimizu S, Miyakawa S (October 1996). “Photoallergic drug eruption due to pyridoxine hydrochloride”. The Journal of Dermatology. 23 (10): 708–9. doi:10.1111/j.1346-8138.1996.tb02685.x. PMID 8973037. S2CID 28810619.
External links
- Media related to Pyritinol at Wikimedia Commons
| Clinical data | |
|---|---|
| ATC code | N06BX02 (WHO) |
| Pharmacokinetic data | |
| Elimination half-life | 2.5 hours |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 1098-97-1 |
| PubChem CID | 14190 |
| ChemSpider | 13561 |
| UNII | AK5Q5FZH2R |
| KEGG | D02160 |
| ChEMBL | ChEMBL488093 |
| CompTox Dashboard (EPA) | DTXSID3048362 |
| ECHA InfoCard | 100.012.864 |
| Chemical and physical data | |
| Formula | C16H20N2O4S2 |
| Molar mass | 368.473 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |
//////////////Pyritinol, пиритинол , بيريتينول , 吡硫醇 , Nootropic,
