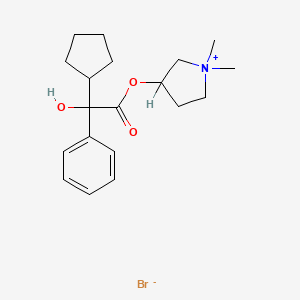
Glycopyrronium bromide
Cas 596-51-0,
- 3-Hydroxy-1,1-dimethylpyrrolidinium bromide α-cyclopentylmandelate (6CI,7CI)
- Pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-, bromide (9CI)
- Pyrrolidinium, 3-hydroxy-1,1-dimethyl-, bromide, α-cyclopentylmandelate (8CI)
- 1,1-Dimethyl-3-hydroxypyrrolidinium bromide α-cyclopentylmandelate
- AHR-504
- Asecryl
- Copyrrolate
- Gastrodyn
- Glycopyrrolate
- Glycopyrrolate bromide
- Glycopyrrone bromide
- Glycopyrronium bromide
- NSC 250836
- NSC 251251
- NSC 251252
- NVA 237
- Nodapton
- Robanul
- Robinul
- Seebri
- Tarodyl
- Tarodyn
- β-1-Methyl-3-pyrrolidyl-α-cyclopentylmandelate methobromide
CAS FREE FORM OF ABOVE 13283-82-4
Glycopyrrolate, ATC:A03AB02
- Use:anticholinergic, antispasmodic
- Chemical name:3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide
- Formula:C19H28BrNO3, MW:398.34 g/mol
- EINECS:209-887-0
- LD50:15 mg/kg (M, i.v.); 570 mg/kg (M, p.o.);
709 mg/kg (R, p.o.)
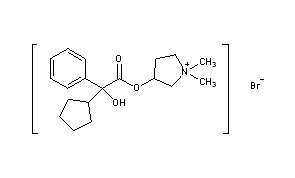
- Pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-, bromide, (R*,S*)-(±)-
- Pyrrolidinium, 3-[[(2R)-cyclopentylhydroxyphenylacetyl]oxy]-1,1-dimethyl-, bromide, (3S)-rel- (9CI)
- erythro-Glycopyrronium bromide
FREE FORM OF ABOVE 740028-90-4
NMR analysis of the diastereomers of glycopyrronium bromide
Finnish Chemical Letters (1975), (3-4), 94-6
Michael Woehrmann, Lara Terstegen, Stefan Biel, Thomas Raschke, Svenja-Kathrin Cerv, Werner Zilz, Sven Untiedt, Thomas Nuebel, Uwe Schoenrock, Heiner Max, Helga Biergiesser, Yvonne Eckhard, Heike Miertsch, Heike Foelster, Cornelia Meier-Zimmerer, Bernd Traupe, Inge Kruse, “GLYCOPYRROLATE IN COSMETIC PREPARATIONS.” U.S. Patent US20090208437, issued August 20, 2009.US20090208437

Glycopyrrolate is a muscarinic antagonist used as an antispasmodic, in some disorders of the gastrointestinal tract, and to reduce salivation with some anesthetics.
Glycopyrronium (as the bromide salt glycopyrrolate) is a synthetic anticholinergic agent with a quaternary ammonium structure. A muscarinic competitive antagonist used as an antispasmodic, in some disorders of the gastrointestinal tract, and to reduce salivation with some anesthetics. In October 2015, glycopyrrolate was approved by the FDA for use as a standalone treatment for Chronic obstructive pulmonary disease (COPD), as Seebri Neohaler.
Medical uses
In anesthesia, glycopyrronium injection can be used as a before surgery in order to reduce salivary, tracheobronchial, and pharyngealsecretions, as well as decreasing the acidity of gastric secretion. It is also used in conjunction with neostigmine, a neuromuscular blocking reversal agent, to prevent neostigmine’s muscarinic effects such as bradycardia.
It is also used to reduce excessive saliva (sialorrhea),[3][4][5] and Ménière’s disease.[6]
It decreases acid secretion in the stomach and so may be used for treating stomach ulcers, in combination with other medications.
It has been used topically and orally to treat hyperhidrosis, in particular, gustatory hyperhidrosis.[7][8]
In inhalable form it is used to treat chronic obstructive pulmonary disease (COPD). Doses for inhalation are much lower than oral ones, so that swallowing a dose will not have an effect.[9][10]
Side effects
Since glycopyrronium reduces the body’s sweating ability, it can even cause hyperthermia and heat stroke in hot environments. Dry mouth, difficulty urinating, headaches, diarrhea and constipation are also observed side effects of the medication. The medication also induces drowsiness or blurred vision, an effect exacerbated by the consumption of alcohol.
Pharmacology
Mechanism of action
Glycopyrronium blocks muscarinic receptors,[11] thus inhibiting cholinergic transmission.
Pharmacokinetics
Glycopyrronium bromide affects the gastrointestinal tracts, liver and kidney but has a very limited effect on the brain and the central nervous system. In horse studies, after a single intravenous infusion, the observed tendencies of glycopyrronium followed a tri-exponential equation, by rapid disappearance from the blood followed by a prolonged terminal phase. Excretion was mainly in urine and in the form of an unchanged drug. Glycopyrronium has a relatively slow diffusion rate, and in a standard comparison to atropine, is more resistant to penetration through the blood-brain barrier and placenta.[12]
Research
It has been studied in asthma.[13][14]

Synthesis
PATENT
https://patents.google.com/patent/CN103819384A/en


PAtent
https://patents.google.com/patent/CN103159659A/en

glycopyrrolate (I)
Methyl ethyl ketone (20mL) IOOmL three-necked flask was added 8 (4.6g, 15mmol) was, at (Γ5 ° C was added dropwise dibromomethane (2.9g, 30mmol) in butanone (5 mL) was added dropwise completed, continued The reaction was stirred for 15min, and a white solid precipitated, was allowed to stand 36h at room temperature, filtered off with suction, the filter cake was sufficiently dried to give crude ketone was recrystallized twice to give a white powdery crystals I (3.9g, 66%) mp 191~193 ° C chromatographic purity 99.8% [HPLC method, mobile phase: lmol / L triethylamine acetate – acetonitrile – water (1: 150: 49); detection wavelength: 230nm, a measurement of the area normalization method] .MS m / z: 318 ( m-BrO 1HNMR (CD3OD) δ:! 1.33~1.38 (m, 2H), 1.55~1.70 (m, 6H), 2.11~2.21 (m, 1H), 2.67~2.80 (m, 1H), 3.02 (m, 1H), 3.06 (s, 3H), 3.23 (s, 3H), 3.59~3.71 (m, 3H), 3.90 (dd, /=13.8,1H), 5.47 (m, 1H), 7.27 (t, 1H) , 7.35 (t, 2H), 7.62 (dd, 2H) .13C bandit R (DMSO) δ: 27.0, 27.4, 28.0, 31.3, 47.8, 53.8, 54.3, 66.0, 71.3, 74.6, 81.1, 126.9,128.7,129.3 , 143.2 17 5.00
Patent
https://patents.google.com/patent/WO2016204998A1/en

PATENT
https://patents.google.com/patent/EP2417106B1/en
-
Glycopyrronium bromide, also known as 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide or glycopyrrolate, is an antimuscarinic agent that is currently administered by injection to reduce secretions during anaesthesia and or taken orally to treat gastric ulcers.
- [0003]
- [0004]
United States patent US 2,956,062 discloses that 1-methyl-3-pyrrolidyl alpha-cyclopentyl mandelate and can be prepared from methyl alpha cyclopentylmandelate and that the methyl bromide quaternary salt can be prepared by saturating a solution of 1-methyl-3-pyrrolidyl alpha-cyclopentyl mandelate in dry ethyl acetate with methyl bromide and filtering the crystalline solid that appears on standing.
- [0005]
The process of US 2,956,062 for preparing 1-methyl-3-pyrrolidyl alpha-cyclopentyl mandelate involves transesterifying methyl glycolate with an amino alcohol under the influence of metallic sodium to give a glycolate intermediate. Metallic sodium is highly reactive, which poses health and safety risks that make its use undesirable on an industrial scale for commercial manufacture.
- [0006]
The process of US 2,956,062 requires preparing the methylester in a previous step and alkylating the amino esters in a later step to form the desired quaternary ammonium salts.
- [0007]
The process of US 2,956,062 provides a mixture of diastereoisomers. The relative proportions of the diastereoisomers can vary widely between batches. This variation can give rise to surprising differences when preparing dry powder formulations from glycopyrronium bromide, which can cause problems when formulating such dry powders for pharmaceutical use.
- [0008]
United States patent application US 2007/0123557 discloses 1-(alkoxycarbonylmethyl)-1-methylpyrrolidyl anticholinergic esters. It describes coupling (R)-cyclopentylmandelic acid with (R,S)-1-methyl-pyrrolidin-3-ol under Mitsunobu conditions to give pure (R)-stereoisomeric compounds that are reacted with a bromoacetate to give the desired esters. It should be noted however that the chemicals used in Mitsunobu reactions, typically dialkyl azodicarboxylates and triphenylphosphine, pose health, safety and ecological risks that make their use undesirable on an industrial scale for commercial manufacture. They are also generally too expensive to source and too laborious to use in commercial manufacture.
- [0009]
United States patent application US 2006/0167275 discloses a process for the enrichment of the R, R- or S, S-configured glycopyrronium isomers and their thienyl analogues having R, S or S, R configuration.
- [0010]
WO 03/087094 A2 discloses new therapeutically useful pyrrolidinium derivatives, processes for their preparation and pharmaceutical compositions containing them.

EXAMPLE Example 1 Preparation of (3S,2’R)- and (3R,2’S)-3-[(cyclopentyl-hydroxyphenylacetyl)-oxy]-1,1-dimethylpyrrolidinium bromide
- [0071]
30 g of cyclopentyl mandelic acid, dissolved in 135 g dimethylformamide (DMF), were treated with 27 g carbonyldiimidazole at 18°C (in portions) to form the “active amide”. After the addition of 16.9 g of 1-methyl-pyrrolidin-3-ol, the mixture was heated to 60°C within 1 hour and stirred for 18 hours at this temperature. After checking for complete conversion, the mixture was cooled and 200 g water was added. The mixture was extracted with 200 g toluene and the extract was washed with water three times. The organic phase was concentrated to obtain cyclopentyl-hydroxy-phenyl-acetic acid 1-methyl-pyrrolidin-3-yl ester as an about 50% solution in toluene, ready to use for the next step.
- [0072]
This solution was diluted with 120 g of n-propanol and cooled to 0°C. 16.8 g methyl bromide was introduced and the mixture was stirred for 2 hours and then gradually heated to 60°C to evaporate the excess methyl bromide into a scrubber. The mixture was then cooled to 50°C and seed crystals were added to facilitate crystallisation. The temperature was then slowly reduced over 18 hours to 15°C. The solid was then isolated by filtration to obtain 22.7 g after drying. It was composed mainly of one pair of enantiomers, a racemic mixture of (3S,2’R)- and (3R,2’S)-3-[(cyclopentyl-hydroxyphenylacetyl)-oxy]-1,1-dimethylpyrrolidinium bromide, with a purity greater than 90% (by HPLC). The other pair of diastereoisomers ((3R,2’R)- and (3S,2’S)-3-[(cyclopentyl-hydroxyphenyl-acetyl)-oxyl-1,1-dimethylpyrrolidinium bromide) remains mainly in the filtrate as those compounds are significantly more soluble in n-propanol than the other stereoisomers.
- [0073]
The solid obtained is further recrystallised in n-propanol (1:10 wt) to give pure (3S,2’R)- and (3R,2’S)-3-[(cyclopentyl-hydroxyphenylacetyl)-oxy]-1,1-dimethylpyrrolidinium bromide i.e. purity > 99.9% as determined by high performance liquid chromatography (HPLC).
- [0074]
Reference Example 2 Preparation of cyclopentyl-hydroxy-phenyl-acetic acid 1-methyl-pyrrolidin-3yl-ester in toluene
- [0075]
1 g of cyclopentyl mandelic acid was suspended in 4.7 g of toluene and 1.5 g of carbonyldiimidazole were added as a solid. After 30 minutes 0.69 g of 1-methyl-pyrrolidin-3-ol and 20 mg of sodium tert-butylate were added. The mixture was stirred at room temperature for 18 hours then water was added. After stirring the phases were separated and the organic phase was washed with water twice and evaporated to obtain an approximately 50% solution of cyclopentyl-hydroxy-phenyl-acetic acid 1-methyl-pyrrolidin-3yl-ester in toluene.
Example 3 Preparation of 2-cyclopentyl-2-hydroxy-1-imidazol-1-yl-2-phenyl-ethanone, the active intermediate
- [0076]
The imidazolidyl derivative of cyclopentylmandelic acid was prepared and isolated as a solid by the following method:
- [0077]
10 g of cyclopentylmandelic acid were suspended in 30 ml of acetonitrile and the mixture was cooled to 0°C. 10.3 g of carbonyldiimidazole were added as a solid and the mixture was warmed to room temperature for 2 hours. Carbon dioxide evolved as a gas as a precipitate formed. The mixture was then cooled to 5°C and the solid was filtered, washed with acetonitrile and dried in vacuum at 40°C to obtain 7.3 g of pure 2-cyclopentyl-2-hydroxy-1-imidazol-1-yl-2-phenyl-ethanone.
- [0078]
- [0079]
High resolution MS-spectroscopy revealed the molecular formula of the compound (as M+H) to be C16H19O2N2 with an exact mass of 271.14414 (0.14575ppm deviation from the calculated value).
1H-NMR-spectroscopy (600MHz, DMSO-d6): 1.03-1.07 (m, 1H), 1.25-1.30 (m, 1H), 1.35-1.40 (m, 1H), 1.40-1.50 (m, 1H), 1.53-1.56 (m, 2H), 1-60-1.67 (m, 1H), 1.75-1.84 (m, 1H), 1.03 – 1.85 (8H, 8 secondary CH2-protons in the cyclopentylring, H-C11, H-C12, H-C13, H-C14); 2.7-2.9 (m, 1H, H-C10); 6.76 (1H, H-C5); 6.91 (1H, H-C4); 7.29 (1H, H-C18); 7.39 (2H, H-C17, H-C19); 7.49 (2H, H-C16, H-C20); 7.65 (1H, H-C2). - [0080]
The compound was characterised by IR-spectroscopy (measured as a solid film on a BRUKER TENSOR 27 FT-IR spectrometer over a wave number range of 4000-600 cm-1 with a resolution of 4 cm-1). An assignment of the most important bands is given below:
Wavenumber (cm-1) Assignments 3300 ∼ 2500 O-H stretching 3167, 3151, 3120 Imidazole CH stretching 2956, 2868 Cyclopentyl CH stretching 1727 C=O stretching 1600, 1538, 1469 Aromatic rings stretching 735 Mono-subst. benzene CH o.o.p. bending 704 Mono-subst. benzene ring o.o.p. bending
SYN
PAPER
https://link.springer.com/article/10.1007/s41981-018-0015-4
Journal of Flow Chemistry, pp 1–8| Cite as
Sequential α-lithiation and aerobic oxidation of an arylacetic acid – continuous-flow synthesis of cyclopentyl mandelic acid

The medicinal properties of glycopyrronium bromide (glycopyrrolate, 4) were first identified in the late 1950s [1]. Glycopyrrolate is an antagonist of muscarinic cholinergic receptors and is used for the treatment of drooling or excessive salivation (sialorrhea) [2], excess sweating (hyperhidrosis) [3], and overactive bladder and for presurgery treatment. In addition, it has recently been introduced as an effective bronchodilator for the treatment of chronic obstructive pulmonary disease (COPD) for asthma patients [4]. Glycopyrrolate displays few side effects because it does not pass through the blood brain barrier. Cyclopentyl mandelic acid (CPMA, 1), or its corresponding ester derivatives, are key intermediates in the synthetic routes to 4. CPMA (1) reacts with 1-methyl-pyrrolidin-3-ol (2) to form tertiary amine 3. N-Methylation of 3 by methyl bromide gives quaternary ammonium salt glycopyrrolate 4 as a racemate (Scheme 1) [5].
Scheme 1
Synthesis of glycopyrrolate 4 from CPMA (1)
CPMA (1) is a synthetically challenging intermediate to prepare (Scheme 2). Routes A to D are most likely to be the commercially applied methods because these procedures are described in patents [5]. The published descriptions for the yields of 1 range from 28 to 56% for routes A to D. Ethyl phenylglyoxylate is reacted with cyclopentyl magnesium bromide to form an ester which is then hydrolyzed (route A) [6]. Phenylglyoxylic acid can be reacted in a similar manner with cyclopentyl magnesium bromide to directly form 1 (route B) [7]. Alternatively, the inverse addition of phenyl-Grignard reagent to cyclopentyl glyoxylic acid ester is reported (route C) [8]. Cyclopentyl glyoxylic acid ester can also be reacted with cyclopentadienyl magnesium bromide which is followed by an additional hydrogenation step with Pd/C and H2 to afford 1 (route D) [9, 10].
Scheme 2
Existing synthetic pathways to CPMA (1)
PATENT
EXA M PL E S
EXAM PL E 1
Scheme 1
ST E P I
To a stirred solution of N-methyl pyrrol i din- 3-ol (2, 1 equiv) and Et3N (1.2 equiv) in dichloromethane was added a solution of 2-cyclopentyl-2-oxoacetyl chloride (1, 1.1 equiv) in DCM at O °C under nitrogen atmosphere for 20 min. The resulting solution was allowed to stir at room temperature over 10h. After completion, the mixture was quenched with water and extracted with diethyl ether to afford the pure product (3A).
Similarly, the product 3A is also obtained by reaction of 2 with other reagents, phenyl oxalic acid, methyl phenyl oxalate, and phenyl hemi-oxaldehyde respectively as shown in Scheme 1.
ST E P II
3A
To a mixture of bromobenzene (2.2 equiv) and Mg metal (2.2 equiv) in TH F (15 mL) was stirred over a period of 30 min at 0 · C. To this mixture, a solution of 1 -methyl pyrrol idin-3-yl 2-cyclopentyl-2-oxoacetate (3, 1 equiv) in T HF was added in portions over a period of 30 min. Up on completion, the reaction mixture was poured into ice water and extracted with ethyl acetate. The organic layer was separated and concentrated in vacuo. The resulting residue was purified by column chromatography to afford the pure product (5).
ST E P III
To a solution of compound 5 (1 equiv) in acetonitrile and chloroform mixture (10 mL, 2:3) was added methyl bromide (4 equiv). The mixture was stirred at room temperature for 72h. The solvents were evaporated, and the resulting residue was washed with diethyl ether to afford the pure product (6) as a white solid.
EXAM PL E 2
Scheme 2
ST E P I
To a stirred solution of N-methyl pyrrol i din- 3-ol (2, 1 equiv) and Et3N (1.2 equiv) in dichloromethane was added a solution of 2- oxo-2- phenyl acetyl chloride (1.1 equiv) in dichloromethane at 0 °C under nitrogen atmosphere for 15 min. The resulting solution was allowed to stir at room temperature over 12h. After completion, the mixture was quenched with water and extracted with diethyl ether to afford the pure product (3B).
Similarly, the product 3B is also obtained by reaction of 2 with other reagents, phenyl oxalic acid, methyl phenyl oxalate, and phenyl hemi-oxaldehyde respectively as shown in Scheme 2.
ST E P II
To a mixture of cyclopentyl bromide (4, 2.2 equiv) and Mg metal (2.2 equiv) in THF (15 mL) was stirred over a period of 30 min at 0 – C. To this mixture, a solution of 1-methylpyrrolidin-3-yl-2-oxo-2-phenylacetate (3B, 1 equiv) in TH F was added in portions over a period of 30 min. Up on completion, the reaction mixture was poured into ice water and extracted with ethyl acetate. The organic layer was separated and concentrated in vacuo. The resulting residue was purified by column chromatography to afford the pure product (5).
ST E P III
To a solution of compound 5 (1 equiv) in acetonitrile and chloroform mixture (10 mL, 2:3) was added methyl bromide (4 equiv). The mixture was stirred at room temperature for 75h. The solvents were evaporated, and the resulting residue was washed with diethyl ether to afford the pure product (6) as a white solid.
The invention has been described in detail with reference to preferred embodiments thereof. However, it will be appreciated by those skilled in the art that changes may be made in these embodiments without departing from the principles and nature of the invention, the scope of which is defined in the appended claims and their equivalents.
References
- Jump up^ Bajaj V, Langtry JA (July 2007). “Use of oral glycopyrronium bromide in hyperhidrosis”. Br. J. Dermatol. 157 (1): 118–21. doi:10.1111/j.1365-2133.2007.07884.x. PMID 17459043.
- Jump up^ “FDA OKs first drug made to reduce excessive sweating”. AP News. Retrieved 2018-07-02.
- Jump up^ Mier RJ, Bachrach SJ, Lakin RC, Barker T, Childs J, Moran M (December 2000). “Treatment of sialorrhea with glycopyrrolate: A double-blind, dose-ranging study”. Arch Pediatr Adolesc Med. 154 (12): 1214–8. doi:10.1001/archpedi.154.12.1214. PMID 11115305.
- Jump up^ Tscheng DZ (November 2002). “Sialorrhea – therapeutic drug options”. Ann Pharmacother. 36 (11): 1785–90. doi:10.1345/aph.1C019. PMID 12398577.[permanent dead link]
- Jump up^ Olsen AK, Sjøgren P (October 1999). “Oral glycopyrrolate alleviates drooling in a patient with tongue cancer”. J Pain Symptom Manage. 18 (4): 300–2. doi:10.1016/S0885-3924(99)00080-9. PMID 10534970.
- Jump up^ Maria, Sammartano Azia; Claudia, Cassandro; Pamela, Giordano; Andrea, Canale; Roberto, Albera (1 December 2012). “Medical therapy in Ménière’s disease”. Audiological Medicine. 10 (4): 171–177. doi:10.3109/1651386X.2012.718413 – via Taylor and Francis+NEJM.
- Jump up^ Kim WO, Kil HK, Yoon DM, Cho MJ (August 2003). “Treatment of compensatory gustatory hyperhidrosis with topical glycopyrrolate”. Yonsei Med. J. 44 (4): 579–82. doi:10.3349/ymj.2003.44.4.579. PMID 12950111.
- Jump up^ Kim WO, Kil HK, Yoon KB, Yoon DM (May 2008). “Topical glycopyrrolate for patients with facial hyperhidrosis”. Br. J. Dermatol. 158 (5): 1094–7. doi:10.1111/j.1365-2133.2008.08476.x. PMID 18294315.
- Jump up^ “EPAR – Product information for Seebri Breezhaler” (PDF). European Medicines Agency. 28 September 2012.
- Jump up^ Tzelepis G, Komanapolli S, Tyler D, Vega D, Fulambarker A (January 1996). “Comparison of nebulized glycopyrrolate and metaproterenol in chronic obstructive pulmonary disease”. Eur. Respir. J. 9 (1): 100–3. doi:10.1183/09031936.96.09010100. PMID 8834341.
- Jump up^ Haddad EB, Patel H, Keeling JE, Yacoub MH, Barnes PJ, Belvisi MG (May 1999). “Pharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and guinea-pig airways”. Br. J. Pharmacol. 127 (2): 413–20. doi:10.1038/sj.bjp.0702573. PMC 1566042
![Freely accessible Freely accessible]() . PMID 10385241.
. PMID 10385241. - Jump up^ Rumpler, M.J.; Colahan, P.; Sams, R.A. (2014). “The pharmacokinetics of glycopyrrolate in Standardbred horses”. J. Vet Pharmacol Ther. 37 (3): 260–8. doi:10.1111/jvp.12085. PMID 24325462.
- Jump up^ Hansel TT, Neighbour H, Erin EM, et al. (October 2005). “Glycopyrrolate causes prolonged bronchoprotection and bronchodilatation in patients with asthma”. Chest. 128 (4): 1974–9. doi:10.1378/chest.128.4.1974. PMID 16236844.
- Jump up^ Gilman MJ, Meyer L, Carter J, Slovis C (November 1990). “Comparison of aerosolized glycopyrrolate and metaproterenol in acute asthma”. Chest. 98 (5): 1095–8. doi:10.1378/chest.98.5.1095. PMID 2225951.
 |
|
| Clinical data | |
|---|---|
| Trade names | Robinul, Cuvposa, Seebri, Qbrexza, others |
| License data | |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.008.990  |
| Chemical and physical data | |
| Formula | C19H28BrNO3 |
| Molar mass | 398.335 g/mol |
| 3D model (JSmol) | |
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602014 |
| Pregnancy category |
|
| Routes of administration |
By mouth, intravenous, inhalation |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 0.6–1.2 hours |
| Excretion | 85% renal, unknown amount in the bile |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.008.990  |
| Chemical and physical data | |
| Formula | C19H28NO3+ |
| Molar mass | 318.431 g/mol |
| 3D model (JSmol) | |