
Sodium zirconium cyclosilicate
ZS-9, ZS 9, UZSi-9
CAS 242800-27-7, H2 O3 Si . x H2 O . 2/3 Na . 1/3 Zr, Sodium zirconium cyclosilicate; Silicic acid (H2SiO3), Sodium zirconium(4+) salt (3:2:1), hydrate
USAN CAS 17141-74-1, H6 O9 Si3 . 2 Na . Zr, Silicic acid (H2SiO3), sodium zirconium(4+) salt (3:2:1), hydrate, Sodium zirconium silicate (Na2ZrSi3O9) hydrate
ナトリウムジルコニウムシクロケイ酸塩
ZrH4O6. 3H4SiO4. 2H2O. 2Na, 561.6068, AS IN kegg
Molecular Formula, H6-O9-Si3.2Na.Z, Molecular Weight, 371.5004 as in chemid plus
APPROVED FDA 2018/5/18, LOKELMA, NDA 207078
APPROVED EMA 2018/3/22, LOKELMA
ATC code: V03AE10
UNII-D652ZWF066
| TREATMENT |
selective cation exchanger
Treatment of hyperkalemia |
|---|
Sodium zirconium cyclosilicate (ZS-9) is a selective oral sorbent that traps potassium ions throughout the gastrointestinal tract. It is being developed by ZS Pharma and AstraZeneca for the treatment of hyperkalemia (elevated serum potassium levels).[1]
The product was originated at ZS Pharma, a wholly owned subsidiary of AstraZeneca. In 2015, ZS Pharma was acquired by AstraZeneca.
Hyperkalaemia is the presence of an abnormally high concentration of potassium in the blood. Most data on the occurrence of hyperkalaemia have been obtained from studies of hospitalised patients, and the incidence ranges from 1 to 10%. There is no agreed definition of hyperkalaemia, since the raised level of potassium at which a treatment should be initiated has not been established. The European Resuscitation Council guidelines consider hyperkalaemia to be a serum potassium (S-K) level > 5.5 mmol/L, with mild elevations defined as 5.5 to 5.9 mmol/L, moderate as 6.0-6.4 mmol/L, and severe as ≥ 6.5 mmol/L. The guidelines also note that extracellular potassium levels are usually between 3.5 and 5.0 mmol/L, which is considered the normal range for adults. However, a number of recent retrospective studies have shown the risk of mortality is increased even with only modest elevations of S-K. Mortality risk has been shown to be significantly higher in chronic kidney disease (CKD) patients with S-K levels > 5.0 mmol/L. In acute myocardial infarction patients, a mean postadmission S-K ≥ 5.5 mmol/L during hospitalisation corresponded to a 12-fold increase in death compared with S-K levels between 3.5 and 4.5 mmol/L but, more importantly, S-K levels between 4.5 and 5.0 mmol/L, which is within the normal range, were associated with a 2-fold increased risk of mortality compared with S-K between 3.5 and 4.5 mmol/L.
Sodium zirconium cyclosilicate (ZS) has been developed as treatment for hyperkalaemia. The indication applied for is: Treatment of hyperkalaemia in adult patients, acute and extended use. ZS is an inorganic cation exchange crystalline compound. ZS has a high capacity to selectively entrap monovalent cations, specifically excess potassium and ammonium ions, over divalent cations such as calcium and magnesium, in the gastrointestinal tract. The high specificity of ZS for potassium is attributable to the chemical composition and diameter of the micro pores, which act in an analogous manner to the selectivity filter utilized by physiologic potassium channels. The exchange with potassium ions occurs throughout the gastrointestinal tract with onset in the upper part of the gastrointestinal tract. The trapped potassium ions are excreted from the body via the faeces, thereby reducing any excess and resolving hyperkalaemia. As claimed by the applicant, ZS demonstrates improved capacity, selectivity, and speed for entrapping excess potassium over currently available options for the treatment of hyperkalaemia. The proposed commercial formulation of ZS is a non-absorbed, insoluble, white crystalline powder for suspension with a specific particle size distribution profile. The proposed starting dose of ZS for reversal of hyperkalaemia (when serum potassium is > 5.0 mmol/l) is up to 10 g/day, divided in 3 doses (TID) to achieve normokalaemia.
EMA
The chemical name of the active substance is hydrogen sodium zirconium (IV) silicate hydrate. Due to the natural variability in the manufacturing process of the active substance, it is expected to have the formula Na~1.5H~0.5ZrSi3O9 • 2–3 H2O and relative molecular mass in the range of 390.5 – 408.5. The WHO chose not to designate an INN for the active substance, and a USAN sodium zirconium cyclosilicate is used throughout the dossier and this CHMP AR. The active substance has the following structure:
Figure 1. Stick-and-ball (left) and polyhedral (right) unit cell structural representation of the main framework of the microporous sodium zirconium cyclosilicate active substance. Red = zirconium, green = silicon, blue = oxygen atoms. Cations are not pictured.
The structure of sodium zirconium cyclosilicate is a cubic cell arrangement of octahedrally coordinated Zr and tetrahedrally coordinated Si units that interconnect through oxygen bridges as Zr–O–Si and Si–O–Si. The two types of units are observed in a ratio 1:3, respectively, and repeat orderly to form a three-dimensional framework characteristic of the compound. The framework acquires its negative charge from the octahedral fractions, [ZrO6]2– , and features channels and cavities that interconnect and locate the positive ions that counter-balance the negative charge of the framework. Electrostatic interactions between the framework and the cations allow for mobility and possibility of exchange with other cations that would fit and pass the free pore openings of ~ 3.0 Å. The uniform micropore structure allows a high exchange capacity and selectivity for potassium (K+) and ammonium (NH4 +) cations, providing the compound with its distinctive ion-exchange selectivity features responsible for its mode of action. In vitro characterisation of ion selectivity of sodium zirconium cyclosilicate was provided by the applicant and considered satisfactory
The structure of sodium zirconium cyclosilicate was confirmed using synchrotron powder diffraction, standard X-ray powder diffraction, 29Si magic angle spinning solid nuclear magnetic resonance studies (29Si-MASNMR), Fourier transform infrared spectroscopy, inductive coupled plasma-optical emission spectrometry, wave dispersive X-ray microprobe analysis and thermo-gravimetric analysis. Calculations using proprietary software were also used for structure elucidation. The active substance is a white crystalline powder. Bonding interactions in the main framework are considered primarily of covalent nature, with some ionic contribution due to the difference in electronegativity between Si–O and Zr–O. The covalent bonding interactions in all directions within the crystals make sodium zirconium cyclosilicate a compound insoluble in water or in organic solvents. It is neither hygroscopic nor sensitive to light and it is resistant to heat. During the hydrothermal synthesis, the possibility that other crystalline phases are formed exists. The observed crystalline forms are controlled by the manufacturing process parameters and release specifications. Sodium zirconium cyclosilicate is considered to be a new active substance. The applicant demonstrated that neither it, nor its derivatives have ever been active substances in medicinal products authorised in the EU………http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004029/WC500246776.pdf
TGA
DOC]Australian Public Assessment Report for Sodium zirconium … – TGA
Jan 29, 2018 – The sponsor has submitted an application to register a new chemical entity Lokelma,sodium zirconium cyclosilicate hydrate powder for …
The chemical formula of sodium zirconium cyclosilicate hydrate is Na~1.5H~0.5ZrSi3O9.2-3H2O.

The drug substance ‘sodium zirconium cyclosilicate hydrate’ (abbreviated to ZS) is a white crystalline powder. The structure of ZS is summarised as a cubic cell arrangement of octahedrally coordinated zirconium Zr ([ZrO6]2-) and tetrahedrally coordinated silicon Si ([SiO4]0) units that interconnect through oxygen bridges as Zr-O-Si and Si-O-Si. The two types of units are observed in a ratio of 1:3, respectively, and repeat orderly to form a three dimensional framework characteristic of the compound. The framework acquires its negative charge from the octahedral fractions, [ZrO6]2- and features channels and cavities that interconnect and locate the positive ions (sodium, Na+, and hydrogen, H+) that counter balance the negative charge of the framework.
The manufacturing process is tightly controlled in terms of order of addition of starting material, reaction and crystallisation temperatures, mixing speeds and times, and minimum number of rinses, in order to meet expected yields of the drug substance of an expected quality. In process quality control tests [information redacted] are applied during the manufacturing process to ensure the formation of the correct crystalline structure and batch to batch consistency.
Sodium zirconium cyclosilicate hydrate is completely insoluble.
The drug substance forms part of a family of zirconium silicates that have specific ion exchange properties. Its mechanism of action is based on the cations within its porous crystalline structure, and their ability to freely exchange with a select group of monovalent cations, most specifically the potassium (K+) and ammonium (NH4+) cations. The pore size within the three dimensional crystalline structure has been measured at ~3Å (2.4 x 3.5 Å[1]), which is sufficiently wide enough to trap the potassium monovalent cations which have an approximate ionic diameter of 2.98Å.
The particle size of the drug substance is controlled to maintain a non-systemic mode of action. The sponsor adequately justified not routinely controlling the size of larger particles in the drug substance as differences in particle size were shown to not affect performance as measured by potassium ion exchange capacity (KEC), and there was no correlation between KEC and D90 for clinical lots manufactured.
There are two alternate zirconium silicate crystalline phases which may be formed in the reaction process; Crystalline Phase A (CPA) and Crystalline Phase B (CPB). These layered, two-dimensional structures also exhibit ion exchange properties, although their ion selectivity is less specific for the potassium K+ cations compared to the desired drug substance. PXRD techniques are used to differentiate between the desired drug substance and levels of CPA and CPB. Appropriate limits are applied in the drug substance specification to limit the content of these crystalline phases in the drug substance/drug product.
The quality of the drug substance is controlled by an acceptable specification that includes test and limits for Appearance, Identification (by FTIR and PXRD), KEC , Crystalline Phase A , Crystalline Phase B , Zirconium content , Silicon content , Hafnium content , Moisture content , Particle Size , and Elemental Impurities.
[1] 1 Å = 0.1 nm.

Background
Hyperkalemia occurs in 3 to 10% of hospitalized patients[2] but is often mild. Hyperkalemia can arise from impaired renal function, potassium-sparing diuretics and renin–angiotensin system blockers (e.g., ACE inhibitors, angiotensin receptor blockers, spironolactone) and diabetes mellitus.[2][3][4][5]
There is no universally accepted definition of what level of hyperkalemia is mild, moderate, or severe.[6] However, if hyperkalemia causes any ECG change it is considered a medical emergency[6] due to a risk of potentially fatal abnormal heart rhythms (arrhythmia) and is treated urgently.[6] serum potassium concentrations greater than 6.5 to 7.0 mmol/L in the absence of ECG changes are managed aggressively.[6]
Hyperkalemia, particularly if severe, is a marker for an increased risk of death.[2] However, there is disagreement regarding whether a modestly elevated serum potassium level directly causes significant problems. One viewpoint is that mild to moderate hyperkalemia is a secondary effect that denotes significant underlying medical problems.[2] Accordingly, these problems are both proximate and ultimate causes of death,[2] and adjustment of potassium may not be helpful. Alternatively, hyperkalemia may itself be an independent risk factorfor cardiovascular mortality.[7]
Several approaches are used in the treatment of hyperkalemia.[6] In October 2015, the U.S. Food and Drug Administration (FDA) approved patiromer which works by binding free potassium ions in the gastrointestinal tract and releasing calcium ions for exchange. Previously, the only approved product was sodium polystyrene sulfonate (Kayexalate),[8] an organic ion-exchange resin that nonspecifically binds cations (e.g., calcium, potassium, magnesium) in the gastrointestinal tract. The effectiveness of sodium polystyrene sulfonate has been questioned: a study in healthy subjects showed that laxatives alone were almost as effective in increasing potassium secretion as laxatives plus Kayexalate.[9] In addition, use of sodium polystyrene sulfonate, particularly if formulated with high sorbitol content, is uncommonly but convincingly associated with colonic necrosis.[6][8][10][11]
Mechanism of action
Cross-sections of ZS-9 pores with three different ions (K⁺ = potassium, Na⁺ = sodium, Ca²⁺ = calcium). The specificity for potassium is thought to be caused by the diameter and composition of the pores, which resembles potassium channels.
ZS-9 is a zirconium silicate. Zirconium silicates have been extensively used in medical and dental applications because of their proven safety.[12] 11 zirconium silicates were screened by an iterative optimization process. ZS-9 selectively captures potassium ions, presumably by mimicking the actions of physiologic potassium channels.[13] ZS-9 is an inorganic cation exchanger crystalline with a high capacity to entrap monovalent cations, specifically potassium and ammonium ions, in the GI tract. ZS-9 is not systemically absorbed; accordingly, the risk of systemic toxicity may be minimized.
Clinical studies
A phase 2 clinical trial in 90 patients with chronic kidney disease and mild-to-moderate hyperkalemia found a significantly greater reduction in serum potassium with ZS-9 than placebo. ZS-9 was well tolerated, with a single adverse event (mild constipation).[14]
A double-blind, phase 3 clinical trial in 753 patients with hyperkalemia and underlying chronic kidney disease, diabetes, congestive heart failure, and in patients on renin–angiotensin system blockers compared ZS-9 with placebo.[15] Patients were randomly assigned to receive either ZS-9 (1.25 g, 2.5 g, 5 g, or 10 g) or placebo 3 times daily for 48 hours (acute phase). Patients who achieved normokalemia (serum potassium of 3.5-4.9 mmol/L) were randomly assigned to receive ZS-9 or placebo once daily for 12 additional days (maintenance phase). At the end of the acute phase, serum potassium significantly decreased in the 2.5 g, 5 g, and 10 g ZS-9 groups. During the maintenance phase, once daily 5 g or 10 g ZS-9 maintained serum potassium at normal levels. Adverse events, including specifically gastrointestinal effects, were similar with either ZS-9 or placebo.[15]
A double-blind, phase 3 clinical trial in 258 patients with hyperkalemia and underlying chronic kidney disease, diabetes, congestive heart failure, and in patients on renin–angiotensin system blockers compared ZS-9 with placebo.[16] All patients received 10 g ZS-9 three times daily for 48 hours in the initial open-label phase. Patients who achieved normokalemia (serum potassium 3.5-5.0 mEq/L) were randomly assigned to receive either ZS-9 (5 g, 10 g, or 15 g) or placebo once daily for 28 days (double-blind phase). 98% of patients (n=237) achieved normokalemia during the open-label phase. During the double-blind phase, once daily 5 g, 10 g, and 15 g ZS-9 maintained serum potassium at normal levels in a significantly higher proportion of patients (80%, 90%, and 94%, respectively) than placebo (46%). Adverse events were generally similar with either ZS-9 or placebo. Hypokalemiaoccurred in more patients in the 10 g and 15 g ZS-9 groups (10% and 11%, respectively), versus none in the 5 g ZS-9 or placebo groups.[16]
Regulatory
In the United States, regulatory approval of ZS-9 was rejected by the Food and Drug Administration in May 2016 due to issues associated with manufacturing.[17] On May 18th, 2018, the FDA approved ZS-9 (now known as Lokelma®) for treatment of adults with hyperkalemia.[18]
PATENT
WO 2012109590
PATENT
WO 2015070019
https://patents.google.com/patent/WO2015070019A1/en
The present invention relates to novel zirconium silicate (“ZS”) compositions which are preferably sodium zirconium cyclosilicates having an elevated level of ZS-9 crystalline form relative to other forms of zirconium cyclosilicates (i.e., ZS-7) and zirconium silicates (i.e., ZS-8, ZS-11). The ZS compositions are preferably sodium zirconium cyclosilicate compositions where the crystalline form has at least 95% ZS-9 relative to other crystalline forms of zirconium silicate. The ZS compositions of the present invention unexpectedly exhibit a markedly improved in vivo potassium ion absorption profile and rapid reduction in elevate levels of serum potassium.
[004] Preferably ZS compositions of the present invention are specifically formulated at particular dosages to remove select toxins, e.g., potassium ions or ammonium ions, from the gastrointestinal tract at an elevated rate without causing undesirable side effects. The preferred formulations are designed to remove and avoid potential entry of particles into the bloodstream and potential increase in pH of urine in patients. The formulation is also designed to release less sodium into the blood. These compositions are particularly useful in the therapeutic treatment of hyperkalemia and kidney disease. The present invention also relates to pharmaceutical granules, tablets, pill, and dosage forms comprising the microporous ZS as an active ingredient. In particular, the granules, tablets, pills or dosage forms are compressed to provide immediate release, delayed release, or specific release within the subject. Also disclosed are microporous ZS compositions having enhanced purity and potassium exchange capacity (“KEC”). Methods of treating acute, sub-acute, and chronic hyperkalemia have also been investigated. Disclosed herein are particularly advantageous dosing regimens for treating different forms of hyperkalemia using the microporous ZS compositions noted above. In addition, the present invention relates to methods of co-administering microporous ZS compositions in combination with other pharmacologic drugs that are known to induce, cause, or exacerbate the hyperkalemic condition.
Patent
References
- Jump up^ “ZS-9. A selective potassium binder”. ZS-Pharma.
- ^ Jump up to:a b c d e Elliott, M. J.; Ronksley, P. E.; Clase, C. M.; Ahmed, S. B.; Hemmelgarn, B. R. (2010). “Management of patients with acute hyperkalemia”. Canadian Medical Association Journal. 182 (15): 1631–5. doi:10.1503/cmaj.100461. PMC 2952010
![Freely accessible Freely accessible]() . PMID 20855477.
. PMID 20855477. - Jump up^ Stevens, M. S.; Dunlay, R. W. (2000). “Hyperkalemia in hospitalized patients”. International Urology and Nephrology. 32 (2): 177–80. doi:10.1023/A:1007135517950. PMID 11229629.
- Jump up^ Tamirisa, K. P.; Aaronson, K. D.; Koelling, T. M. (2004). “Spironolactone-induced renal insufficiency and hyperkalemia in patients with heart failure”. American Heart Journal. 148(6): 971–8. doi:10.1016/j.ahj.2004.10.005. PMID 15632880.
- ^ Jump up to:a b c d e f Taal, M.W.; Chertow, G.M.; Marsden, P.A.; Skorecki, K.; Yu, A.S.L.; Brenner, B.M. (2012). Brenner and Rector’s The Kidney (Chapter 17, page 672, 9th ed.). Elsevier. ISBN 978-1-4160-6193-9.
- Jump up^ Fang, J.; Madhavan, S.; Cohen, H.; Alderman, M. H. (2000). “Serum potassium and cardiovascular mortality”. Journal of General Internal Medicine. 15 (12): 885–90. doi:10.1046/j.1525-1497.2000.91021.x. PMC 1495719
![Freely accessible Freely accessible]() . PMID 11119186.
. PMID 11119186. - ^ Jump up to:a b Watson, M.; Abbott, K. C.; Yuan, C. M. (2010). “Damned if You Do, Damned if You Don’t: Potassium Binding Resins in Hyperkalemia”. Clinical Journal of the American Society of Nephrology. 5 (10): 1723–6. doi:10.2215/CJN.03700410. PMID 20798253.
- Jump up^ Emmett, M.; Hootkins, R. E.; Fine, K. D.; Santa Ana, C. A.; Porter, J. L.; Fordtran, J. S. (1995). “Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion”. Gastroenterology. 108 (3): 752–60. doi:10.1016/0016-5085(95)90448-4. PMID 7875477.
- Jump up^ Sterns, R. H.; Rojas, M.; Bernstein, P.; Chennupati, S. (2010). “Ion-Exchange Resins for the Treatment of Hyperkalemia: Are They Safe and Effective?”. Journal of the American Society of Nephrology. 21 (5): 733–5. doi:10.1681/ASN.2010010079. PMID 20167700.
- Jump up^ Kamel, K. S.; Schreiber, M. (2012). “Asking the question again: Are cation exchange resins effective for the treatment of hyperkalemia?”. Nephrology Dialysis Transplantation. 27(12): 4294–7. doi:10.1093/ndt/gfs293. PMID 22989741.
- Jump up^ Denry I, Kelly JR. State of the art of zirconia for dental applications. Dental Materials. Volume 24, Issue 3, March 2008, Pages 299–307
- Jump up^ =Stavros, F (2014). “Characterization of Structure and Function of ZS-9, a K⁺ Selective Ion Trap”. PLOS ONE. 9 (12): e114686. doi:10.1371/journal.pone.0114686. PMC 4273971
![Freely accessible Freely accessible]() . PMID 25531770.
. PMID 25531770. - Jump up^ Ash SR, et al. “Safety and efficacy of ZS-9, a novel selective cation trap, for treatment of hyperkalemia in CKD patients.” American Society of Nephrology 2013 conference, Late-Breaking Abstract.
- ^ Jump up to:a b Packham DK, et al. (2014). “Sodium zirconium cyclosilicate in hyperkalemia”. New England Journal of Medicine. 372 (3): 222–31. doi:10.1056/NEJMoa1411487. PMID 25415807.
- ^ Jump up to:a b Kosiborod M, et al. (2014). “Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia”. Journal of the American Medical Association. 312 (21): 2223–33. doi:10.1001/jama.2014.15688. PMID 25402495.
- Jump up^ Ben Adams (May 27, 2016). “AstraZeneca’s $2.7B hyperkalemia drug ZS-9 rejected by FDA”. FierceBiotech.
- Jump up^ https://www.drugs.com/history/lokelma.html

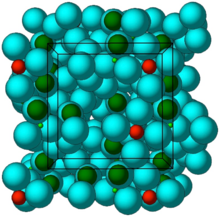
Crystal structure of ZS-9. Blue spheres = oxygen atoms, red spheres = zirconium atoms, green spheres = silicon atoms.
|
|
| Clinical data | |
|---|---|
| Trade names | Lokelma |
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not absorbed |
| Excretion | Stool |
| Identifiers | |
| CAS Number | |
| UNII | |
| KEGG | |
//////////////Sodium zirconium cyclosilicate, ナトリウムジルコニウムシクロケイ酸塩 , FDA 2018, EMA, 2018, EU 2018, ZS 9, UZSi-9
O[Si]1(O[Si](O[Si](O1)(O)O)(O)O)O.[Na+].[Na+].[Zr






