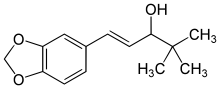
Stiripentol
スチリペントール
STIRIPENTOL; Diacomit; 49763-96-4; BCX 2600; Estiripentol; Stiripentolum
| CAS: | 137767-55-6 49763-96-4 |
(E)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol
| Molecular Formula: | C14H18O3 |
|---|---|
| Molecular Weight: | 234.295 g/mol |
UNII
Stiripentol (marketed as Diacomit by Laboratoires Biocodex) is an anticonvulsant drug used in the treatment of epilepsy. It is approved for the treatment of Dravet syndrome, an epilepsy syndrome. It is unrelated to other anticonvulsants and belongs to the group of aromatic allylic alcohols.
Medical use
It is used in some countries as an add-on therapy with sodium valproate and clobazam for treating children with Dravet syndromewhose seizures are not adequately controlled.[1][2][3] As of 2017 it was not known whether stiripentol remains useful as children become adolescents nor as they become adults.[4]
Adverse effects
Very common (more than 10% of people) adverse effects include loss of appetite, weight loss, insomnia, drowsiness, ataxia, hypotonia, and dystonia.[3]
Common (between 1% and than 10% of people) adverse effects include neutropenia (sometimes severe), aggressiveness, irritability, behavior disorders, opposing behavior, hyperexcitability, sleep disorders, hyperkinesias, nausea, vomiting, and elevated gamma-glutamyltransferase.[3]
Interactions
Stiripentol inhibits several cytochrome P450 isoenzymes and so interacts with many anticonvulsants and other medicines.[3]
Pharmacology
As with most anticonvulsants, the precise mechanism of action is unknown. Regardless, stiripentol has been shown to have anticonvulsant effects of its own.
Stiripentol increases GABAergic activity. At clinically relevant concentrations, it enhances central GABA neurotransmission through a barbiturate-like effect, since it increases the duration of opening of GABA-A receptor channels in hippocampal slices.[5] It has also been shown to increase GABA levels in brain tissues by interfering with its reuptake and metabolism.[6] Specifically, it has been shown to inhibit lactate dehydrogenase, which is an important enzyme involved in the energy metabolism of neurons. Inhibition of this enzyme can make neurons less prone to fire action potentials, likely through activation of ATP-sensitive potassium channels.[7]
Stiripentol also improves the effectiveness of many other anticonvulsants, possibly due to its inhibition of certain enzymes, slowing the drugs’ metabolism and increasing blood plasma levels.[3]
Chemistry
Stiripentol is an α-ethylene alcohol; its chemical formula is 4,4-dimethyl-1-[3,4-(methylendioxy)-phenyl]-1penten-3-ol. It is chiral and is marketed as an equimolar racemic mixture. The R enantiomer appears to be around 2.5 times more active than the S enantiomer.[8]
Paper
Tetrahedron: Asymmetry
Synthesis of the antiepileptic (R)-Stiripentol by a combination of lipase catalyzed resolution and alkene metathesis
The enantiopure (ee >99%) antiepileptic (R)-(+)-Stiripentol has been stereoselectively synthesized via cross metathesis of 5-vinylbenzo[d][1,3]dioxole 1 and (R)-(+)-4,4-dimethylpent-1-en-3-ol (R)-(+)-2. A novel one-pot two-step pathway for the synthesis of 5-vinylbenzo[d][1,3]dioxole 1 starting from 3,4-dihydroxycinnamic acid has been introduced. A lipase catalyzed kinetic resolution access to enantiopure (R)-(+)-4,4-dimethylpent-1-en-3-ol (R)-(+)-2 (ee >99%) has also been developed.

Stiripentol (CAS NO.: 49763-96-4), with other name of 4,4-Dimethyl-1-[(3,4-methylenedioxy)phenyl]-1-penten-3-ol, could be produced through many synthetic methods.
Following is one of the reaction routes:

The synthesis of [14]-labeled stiripentol has been published:The reaction of 3,4-methylenedioxybromobenzene (I) with 14CO2 by means of butyllithium in ether gives 3,4-methylenedioxybenzoic acid (II), which is reduced with LiAlH4 to the corresponding benzyl alcohol (III). Oxidation of (III) with CrO3-pyridine affords the aldehyde (IV), which is condensed with methyl tert-butyl ketone (V) by means of NaOH in refluxing ethanol to give the labeled pentanone (VI). Finally, this compound is reduced to [14C]-labeled stiripentol with NaBH4 in methanol
 |
|
| 合成路线图解说明:The condensation of 3,4-methylenedioxybenzaldehyde (I) with 3,3-dimethyl-2-butanone (II) by means of NaOH in ethanol-water gives 4,4-dimethyl-1-[(3,4-methylenedioxy)phenyl]-1-penten-3-one (III), which is reduced with NaBH4 in methanol. |
 |
|
| 合成路线图解说明:The synthesis of [14]-labeled stiripentol has been published: The reaction of 3,4-methylenedioxybromobenzene (I) with 14CO2 by means of butyllithium in ether gives 3,4-methylenedioxybenzoic acid (II), which is reduced with LiAlH4 to the corresponding benzyl alcohol (III). Oxidation of (III) with CrO3-pyridine affords the aldehyde (IV), which is condensed with methyl tert-butyl ketone (V) by means of NaOH in refluxing ethanol to give the labeled pentanone (VI). Finally, this compound is reduced to [14C]-labeled stiripentol with NaBH4 in methanol. |
History
Stiripentol was discovered in 1978 by scientists at Biocodex and clinical trials started over the next few years.[8] It was originally developed for adults with focal seizures, but failed a Phase III trial.[4]
In December 2001 the European Medicines Agency (EMA) granted stiripentol orphan drug status (designation number EU/3/01/071) for the treatment of severe myoclonic epilepsy of infancy (SMEI, also known as Dravet’s syndrome) in children and in 2007, the EMA granted the drug a marketing authorisation for use of the drug as an add-on to other anti-seizure drugs.[3] It was approved in Canada for this use in 2012.[9] As of 2017 it was also approved for this use in Japan.[2]
As of 2014 it was not approved in the US, and parents of children with Dravets were paying around $1,000 for a month supply to obtain it from Europe.[10]
 |
|
| Clinical data | |
|---|---|
| Trade names | Diacomit |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.051.329  |
| Chemical and physical data | |
| Formula | C14H18O3 |
| Molar mass | 234.30 g·mol−1 |
| 3D model (JSmol) | |
References
- Jump up^ Brigo, F; Igwe, SC; Bragazzi, NL (18 May 2017). “Antiepileptic drugs for the treatment of infants with severe myoclonic epilepsy”. The Cochrane Database of Systematic Reviews. 5: CD010483. doi:10.1002/14651858.CD010483.pub4. PMID 28521067.
- ^ Jump up to:a b Nickels, KC; Wirrell, EC (May 2017). “Stiripentol in the Management of Epilepsy”. CNS drugs. 31 (5): 405–416. doi:10.1007/s40263-017-0432-1. PMID 28434133.
- ^ Jump up to:a b c d e f “Diacomit (stiripentol) SPC” (PDF). EMA. 8 January 2014. Retrieved 1 October 2017. For updates see EMA index page
- ^ Jump up to:a b Nabbout, R; Camfield, CS; Andrade, DM; Arzimanoglou, A; Chiron, C; Cramer, JA; French, JA; Kossoff, E; Mula, M; Camfield, PR (April 2017). “Treatment issues for children with epilepsy transitioning to adult care”. Epilepsy & Behavior. 69: 153–160. doi:10.1016/j.yebeh.2016.11.008. PMID 28188045.
- Jump up^ Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H (2006). “Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels”. Epilepsia. 47 (4): 704–16. doi:10.1111/j.1528-1167.2006.00497.x. PMID 16650136.
- Jump up^ Trojnar MK, Wojtal K, Trojnar MP, Czuczwar SJ (2005). “Stiripentol. A novel antiepileptic drug” (PDF). Pharmacological reports : PR. 57 (2): 154–60. PMID 15886413.
- Jump up^ Sada N, Lee S, Katsu T, Otsuki T, Inoue T (2015). “Targeting LDH enzymes with a stiripentol analog to treat epilepsy”. Science. 347 (6228): 1362–67. doi:10.1126/science.aaa1299. PMID 25792327.
- ^ Jump up to:a b “Scientific evaluation” (PDF). EMA. 2007.
- Jump up^ “Stiripentol (Diacomit): For Severe Myoclonic Epilepsy in Infancy (Dravet Syndrome)” (PDF). Canadian Agency for Drugs and Technologies in Health. April 2015.
- Jump up^ Kossoff, E (January 2014). “Stiripentol for dravet syndrome: is it worth it?”. Epilepsy Currents. 14 (1): 22–3. doi:10.5698/1535-7597-14.1.22. PMC 3913306
![Freely accessible Freely accessible]() . PMID 24526870.
. PMID 24526870.
////////////Stiripentol, fda 2018, Diacomit, 49763-96-4, BCX 2600, Estiripentol, Stiripentolum
CC(C)(C)C(C=CC1=CC2=C(C=C1)OCO2)O




