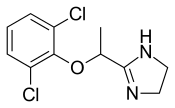
Lofexidine
- Molecular FormulaC11H12Cl2N2O
- Average mass259.132 Da
- (±)-2-[1-(2,6-Dichlorophenoxy)ethyl]-2-imidazoline
FDA Approved May 2018
Lofexidine was developed by US Woldmeds LLC and it got approved by the FDA on May 16, 2018

Experimental Properties
| PROPERTY | VALUE | SOURCE |
|---|---|---|
| melting point (°C) | 221-223 | U.S. Patent 3,966,757. |
| boiling point (°C) | 421.5 ºC at 760 mm Hg | ‘MSDS’ |
| water solubility | Soluble | ‘MSDS’ |
| logP | 5.37 | FDA Advisory Committee Briefing Document. |
| pKa | 9.43 | FDA Advisory Committee Briefing Document. |
SYN
Organic Process Research & Development, 13(3), 415-419; 2009


LOFEXIDINE HYDROCHLORIDE
Cas No. 21498-08-8
Lofexidine, sold under the brand name Lucemyra among others,[1] is a medication historically used to treat high blood pressure, but more commonly used to help with the physical symptoms of opioid withdrawal.[2] It is taken by mouth.[3] It is an α2A adrenergic receptoragonist.[3] It was approved for use by the Food and Drug Administration in the United States in 2018.[3]
Medical uses
In the United States, the brand name Lucemyra (lofexidine HCl) is approved for the “mitigation of withdrawal symptoms to facilitate abrupt discontinuation of opioids in adults,” for a treatment duration of 14 days.[1] In the United Kingdom, lofexidine is commonly used in conjunction with the opioid receptor antagonist naltrexone in rapid detoxification cases. When these two drugs are paired, naltrexone is administered to induce an opioid-receptor blockade sending the subject into immediate withdrawal and accelerating the detoxificationprocess, while lofexidine is given to relieve the symptoms associated with the withdrawal including chills, sweating, stomach cramps, muscle pain, and runny nose.[citation needed]
Opioid withdrawal
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines recommend the use of methadone or buprenorphine as first-line agents in the management of opioid use disorder. However, lofexidine is considered an acceptable alternative for people with mild or uncertain opioid dependence in need of short-term detoxification.[4]
Lofexidine is not an opioid.[3] It does not eliminate the symptoms of opioid withdrawal but reduces them.[3] Indeed, one suggested use for lofexidine is to ease withdrawal symptoms of methadone dependence. Its use is approved in the United States for up to 14 days.[3]
Other clinical uses
The possibility of using lofexidine to treat alcohol withdrawal symptoms has been investigated, and has not yet been shown to be an effective treatment.[5] It is also used in treatment of cases suffering from postmenopausal hot flashes.
Special populations
Lofexidine’s safety in pregnancy or in the setting of breastfeeding are unknown.[6] Caution is warranted if chronic kidney impairment is present.[6]
Adverse effects
Adverse effects that have occurred after taking lofexidine include the following:[6]
In addition, people may experience a sudden jump in blood pressure after stopping lofexidine.[1]
Overdose
The LD50 of lofexidine is above 77 mg/kg in animals. Studies of high-dose, single administrations of lofexidine proved tolerable for animals, but repeat administration induced symptoms consistent with toxicity. In studies on mice, rats, and dogs, these included ataxia, somnolence, and tremors. It is expected that an overdose of lofexidine would result in symptoms akin to its pharmacological side effects in humans, such as bradycardia and hypotension.[7]
Interactions
Many drug-drug interactions with lofexidine are possible.[8]
QT prolongation
Lofexidine prolongs the QT interval, which can result in a severe interaction (torsade de pointes) when combined with other drugs that also prolong the QT interval. Patient-specific characteristics that increase the risk for a clinically-significant drug-drug interaction include:[8]
- increasing age
- female sex
- cardiac disease
- electrolyte disturbances (low blood potassium)
As a result, there are many QT-prolonging drugs that may interact with lofexidine. These include medications such as amiodarone, citalopram, and fluconazole. Other medications may increase the risk for a low level of potassium in the blood, thereby indirectly increasing the risk for QT prolongation. For example, dexamethasone, hydrochlorothiazide, and theophylline can lower the level of potassium in the blood.[8]
CNS depression
Lofexidine can depress the central nervous system (CNS), which, in combination with other CNS depressants, may reduce a person’s ability to perform tasks that require skills and attention. For example, clobazam, gabapentin, and levetiracetam all can depress the CNS.[8]
Hypotension
The risk of hypotension (low blood pressure) is increased when lofexidine is combined with other drugs that lower blood pressure. These may include losartan, metoprolol, and pramipexole.[8]
Pharmacology
Lofexidine is an agonist at the α-2A, 2B, and 2C adrenergic receptor subtypes, with the highest activity at the alpha-2A receptor.[9]
-
-
Ki for lofexidine[9] Adrenergic receptor Ki (nM) α-2A 4 α-2B 67 α-2C 69
-
Ki represents the dissociation constant[10] for lofexidine’s binding to a specific subtype of alpha-2 receptor. The smaller the Ki value, the stronger the drug binds to the receptor to exert its activity.
Lofexidine inhibits the release of norepinephrine in the central and peripheral nervous system, thereby reducing some of the symptoms of opioid withdrawal, but it has no documented effect on drug craving and endogenous opioid levels.[2]
Pharmacokinetics
Lofexidine’s oral bioavailability is about 90%, with extensive oral absorption. Peak plasma concentrations occur at 3 hours after a single administration, with a half-life of 11 hours. Lofexidine is extensively metabolized by the liver, and primarily cleared by the kidney. It is 80-90% plasma protein bound.[7]
Chemistry
Lofexidine exists as a solid at room temperature, with a melting point of 127 degrees C.[7] The pair of ortho chlorine (Cl–) atoms on the phenyl ring are necessary for lofexidine’s agonism at the α2a adrenergic receptor subtype; removal of either chlorine atom results in antagonism at the receptor.[9]
Comparison to clonidine
Lofexidine is structurally analogous to clonidine, another α2 adrenergic receptor agonist used for treatment of opioid withdrawal symptoms. A comparison of the two structures is shown at right. Both contain an imidazoline ring and a 2,6-dichlorinated phenyl ring. The differences in structure are shown in red, while the similarities are in black. In addition to the structural differences, administration of lofexidine to people who abuse opioids has been shown to be more effective for a longer duration, with fewer withdrawal symptoms than clonidine even after one day.[11] However, clonidine is often preferred as it is substantially cheaper than lofexidine when purchased with a private (non-NHS) prescription. This factor is exacerbated by the considerable number of and quantities of medications prescribed to alleviate the constellation of withdrawal signs and symptoms. Additionally, clonidine has been shown to significantly lower blood pressure. Therefore, although similar to lofexidine, clonidine is most frequently prescribed to treat high blood pressure.[citation needed]
Society and culture
Britannia Pharmaceuticals has licensed lofexidine to be sold by US WorldMeds for sale in North America.[12] In the United Kingdom, the hydrochloride form, lofexidine HCl, has been licensed and sold since 1992 for opioid withdrawal relief in tablet form as BritLofex by Britannia Pharmaceuticals.[2] BritLofex is only available by prescription. Lofexidine was first approved by the US FDA on May 16, 2018 under the brand name Lucemyra, produced by US WorldMeds.[13] It was noted as the first, non-opioid drug approved in the US for the treatment of opioid withdrawal.[1]
Heroin has been reported to be the most prominent illicit drug of abuse among admissions at public!} -funded substance abuse treatment facilities in the US. At some time in their lives, about 2.4 million people have used heroin; in 1997, there were 81 ,000 new heroin users of whom 87% were less than 26 years of age. In spite of efforts to decrease illicit drug abuse, the problem escalates and the abusing population is increasingly younger. Hospital emergency room episodes from 21 metropolitan areas show that 14% of drug-related emergency room episodes involved heroin, and such episodes increased more than 2-fold from 1991 to 1996. Additionally, prescription opioid abuse escalates; the number of people addicted to prescription pain relievers is 3 -fold higher than those addicted to heroin. For example, from 1999 to 2001, the non-medical use of OxyContin®increased 4-fold, and its use continues to escalate.
[0003] Generally, opioid addiction has been associated with high morbidity and mortality, with a 15-20 fold increase in risk of death for intravenous drug users compared with their same age peers. Clearly, the medical and social importance of the development of effective treatments for opioid addiction is well recognized. Surprisingly, few treatment options for opioid addiction are available.
[0004] Withdrawal, maintenance and relapse are considered the progressive stages for treatment of opioid addiction. There are two predominant management strategies for the treatment of opioid addiction, detoxification and substitution therapy, which are typically combined with medical, social and psychological support. A majority of individuals may benefit from remaining in the maintenance phase for an indefinite period of time, while others may be able to directly undergo medically-supervised detoxification and/or relapse therapy, without the need for maintenance therapy. Methadone and buprenorphine constitute the most commonly used pharmacotherapies. Although patients continue to be successfully treated with methadone, a mμ opioid receptor agonist, several disadvantages of methadone treatment include the length of time for withdrawal, the difficulty of obtaining complete abstinence, and liability for its abuse. Due to the abuse liability of methadone and its consequent Schedule II classification by the Drug Enforcement Administration (DEA), methadone has additional disadvantages with respect to its prescription requirements, the carefully controlled conditions under which it is dispensed, and the annoyance experienced by patients who must frequently visit the dispensing unit to obtain their methadone dosages.
[0005] BritLofex (Lofexidine hydrochloride 0.2 mg tablet), an α2-adrenergic agonist, is used as a non-opioid medication for opioid detoxification in the United Kingdom (UK). There is no non-opioid medication approved by the Food and Drug Administration (FDA) for this indication in the US. The only medications currently approved by the FDA for opioid detoxification are methadone and buprenorphine, both opioid receptor agonists and both associated with abuse liability. Clonidine, an 012-adrenergic agonist, is often used “off-label” for this indication in the U.S. However, clonidine has not been approved by the FDA for this indication. However, the use of clonidine is limited by its side-effect profile, i.e., significant hypotension at doses effective in alleviating opioid withdrawal symptoms.
(Lofexidine hydrochloride 0.2 mg tablet), an α2-adrenergic agonist, is used as a non-opioid medication for opioid detoxification in the United Kingdom (UK). There is no non-opioid medication approved by the Food and Drug Administration (FDA) for this indication in the US. The only medications currently approved by the FDA for opioid detoxification are methadone and buprenorphine, both opioid receptor agonists and both associated with abuse liability. Clonidine, an 012-adrenergic agonist, is often used “off-label” for this indication in the U.S. However, clonidine has not been approved by the FDA for this indication. However, the use of clonidine is limited by its side-effect profile, i.e., significant hypotension at doses effective in alleviating opioid withdrawal symptoms.
[0006] In contrast, Lofexidine HCl is the only non-opiate, non-addictive treatment approved for use in the UK to manage withdrawal symptoms in patients undergoing opiate detoxification. Lofexidine has been found to be effective in reducing the symptoms associated with heroin withdrawal such as chills, vomiting, sweating, stomach cramps, diarrhea, muscle pain, and runny nose and eyes. In the UK, the treatment is responsible for approximately 20,000 detoxifications per year. The drug’s proven level of safety permits its use in an outpatient situation. This is of great importance to patients in the US who are located in parts of the country where treatment clinics are not readily available.
[0007] Although naltrexone, methadone and more recently buprenorphine are FDA approved in the treatment of opioid addiction, these opioid treatments are associated with high relapse rates. Furthermore, there is currently insufficient availability of methadone and buprenorphine treatment for patients who abuse opioids. A significant number of these patients are undergoing detoxification treatments. However, the great risk of abuse and several other existing restrictions, such as medical prescribing and pharmaceutical dispensing, limit the use of methadone and buprenorphine for outpatient detoxification. In addition, the unapproved status of clonidine, its side effects, such as the lowering of blood pressure, and moderate efficacy limit its use. A substantial amount of research is ongoing to understand the mechanisms that may underline the high rates of relapse associated with opioid addiction. There is growing evidence that chronic drug use results in neuroadaptive changes in brain stress and reward circuits that may be associated with increased drug craving and risk of relapse particularly in the face of environmental triggers such as stressful life events and drug cues.
PATENT
https://patents.google.com/patent/EP2334297A1/en
The lofexidine hydrochloride tablets available in the UK market (BritLofex ) contain the racemic mixture of the drug. However, since lofexidine enantiomers exhibit different affinities for central the nervous system neurotransmitter receptors involved in (±)-lofexidine’s action as a medication for opioid detoxification, each of these enantiomers may have therapeutic benefits in the treatment of opioid addiction.
) contain the racemic mixture of the drug. However, since lofexidine enantiomers exhibit different affinities for central the nervous system neurotransmitter receptors involved in (±)-lofexidine’s action as a medication for opioid detoxification, each of these enantiomers may have therapeutic benefits in the treatment of opioid addiction.
Experimental
[0028] 1) Resolution of (-)-lofexidine and (+)-lofexidine enantiomers found in the racemic mixture using chiral stationary phases by HPLC method:
[0029] A chiral chromatographic matrix was used to separate a racemic mixture of lofexidine into its component enantiomers by a process of HPLC to obtain optically pure (-)- lofexidine and optically pure (+)-lofexidine. The separation was performed using a chiral stationary phase consisted of D-glucose cyclodextran complex (Cyclobond HP-RSP) from Astec
Company (Whippany, NJ, USA) using a mobile phase consisted of 1OmM ammonium acetate
(88%), acetonitrile (8%), and methanol (8%) at 0.85 ml/min flow rate. Analysis was performed using Agilent series 1100 HPLC system comprising a solvent degasser unit, quaternary pump, autosampler, and DAD detector. Using such chiral stationary phase in a preparative scale enables the yield of gram quantities of desired enantiomers.
[0030] Resolution of (-)-lofexidine and (+)-lofexidine enantiomers found in the racemic mixture using a chiral acid, not only diastereomeric salt formation but also preferential crystallization: [0031] Optical resolution of (±)-lofexidine hydrochloride by using the classical methods of salt formation with a chiral acid such as, [( Di-p-toluoyl-D-tartaric acid [D]D20 +142° (c=l, CH3OH)] as shown in Figure 1, yielded (-)-lofexidine hydrochloride and (+)-lofexidine hydrochloride enantiomers (yield = 87%). The method comprised the following steps: [0032] A racemic form of lofexidine (10 mmol) was placed in ethanol (100 mL), and the chiral acid (+)-Di-p-toluoyl-D-tartaric acid was added in order to form a mixture of the (+)(-) and (+)(+) diastereomeric lofexidine salts. The diastereomeric salts i.e.: (+)(-) lofexidine Di-p- toluoyl-D-tartarate salt was separated from the (+)(+) lofexidine Di-p-toluoyl-D-tartarate salt by a process of fractional crystallization. 10 mL methanol and 1 ml water was added and the mixture was heated for 1 hour at 55-65 0C. After the mixture became clear it was left to cool down at room temperature. The crystals were isolated after two days, dried under vacuum. Recrystallization was performed using ethanol (20 volumes). Final yield was 87%. [0033] Chiral purity of the resulting crystals was tested by the chiral HPLC method. The
(+)(-) lofexidine Di-p-toluoyl-D-tartarate salt or the(+)(+) lofexidine Di-p-toluoyl-D-tartarate salt obtained was treated with a base such as 0.1 N sodium carbonate to liberate (-)-lofexidine and (+)-lofexidine. The resulting enantiomerically pure free base of (-)-lofexidine and (+)-lofexidine was converted to lofexidine hydrochloride salt.
PAPER
A Scalable, Enantioselective Synthesis of the α2-Adrenergic Agonist, Lofexidine

A scalable and high-yielding synthetic route toward pure enantiomers of the α2-adrenergic agonist, lofexidine hydrochloride, is presented. Salient features include a rapid one-pot amide alkylation-imidazoline formation sequence on the carboxamide function of α-(2,6-dichlorophenoxy)propionamide, while preserving the sensitive configuration about the α-carbon of the resulting product. A means to accelerate the sluggish O-alkylation of the carboxamide function of α-(2,6-dichlorophenoxy)propionamide by Me3O+BF4− is also described, which may be of general applicability.
PATENTS
US8101779B2 *2008-10-062012-01-24University Of Kentucky Research FoundationEnantioselective synthesis of (+) and (–)-2-[1-(2,6-dichlorophenoxy)-ethyl]-1,3-diazacyclopent-2-ene
References
- ^ Jump up to:a b c d “Press Announcements – FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults”. http://www.fda.gov. U.S. Food and Drug Administration. Retrieved 16 May 2018.
- ^ Jump up to:a b c Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. p. 330. ISBN 978-0-85711-084-8.
- ^ Jump up to:a b c d e f “Press Announcements – FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults”. http://www.fda.gov. Retrieved 18 May2018.
- Jump up^ “Pharmacological interventions in opioid detoxification for drug misuse in people over 16”. pathways.nice.org.uk. NICE. Retrieved 16 May 2018.
- Jump up^ Keaney F, Strang J, Gossop M, Marshall EJ, Farrell M, Welch S, Hahn B, Gonzalez A. A double-blind randomized placebo-controlled trial of lofexidine in alcohol withdrawal: lofexidine is not a useful adjunct to chlordiazepoxide. Alcohol Alcohol (2001) 36:426–30.
- ^ Jump up to:a b c “LOFEXIDINE HYDROCHLORIDE”. bnf.nice.org.uk. NICE. Retrieved 16 May2018.
- ^ Jump up to:a b c “Lofexidine”. pubchem.ncbi.nlm.nih.gov. National Center for Biotechnology Information. Retrieved 16 May 2018.
- ^ Jump up to:a b c d e “Lofexidine | Interactions | BNF”. bnf.nice.org.uk. NICE. Retrieved 16 May 2018.
- ^ Jump up to:a b c Fulton, Brian (2014). Drug Discovery for the Treatment of Addiction: Medicinal Chemistry Strategies. John Wiley & Sons. p. 151. ISBN 0470614161.
- Jump up^ Neubig, R. R. (1 December 2003). “International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology”. Pharmacological Reviews. 55 (4): 597–606. doi:10.1124/pr.55.4.4.
- Jump up^ G. Gerra, et al., Lofexidine versus clonidine in rapid opioid detoxification, Journal of Substance Abuse TreatmentVolume 21, Issue 1, , July 2001, Pages 11-17.
- Jump up^ Britannia Pharmaceuticals Limited
- Jump up^ “Lucemyra (lofexidine hydrochloride) FDA Approval History – Drugs.com”. Drugs.com. Retrieved 16 May 2018.
 |
|
| Clinical data | |
|---|---|
| Trade names | BritLofex, Lucemyra, Kai Er Ding, others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Protein binding | 80–90% |
| Metabolism | Liver (glucuronidation) |
| Elimination half-life | 11 hours |
| Excretion | Kidney |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H12Cl2N2O |
| Molar mass | 259.131 g/mol |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
/////////////lofexidine, FDA 2018, лофексидин , لوفيكسيدين , 洛非西定 , Lofetensin, Loxacor
CC(C1=NCCN1)OC2=C(C=CC=C2Cl)Cl



