Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC01803C, Communication
DOI: 10.1039/C6GC01803C, Communication
Torsten Sehl, Saskia Bock, Lisa Marx, Zaira Maugeri, Lydia Walter, Robert Westphal, Constantin Vogel, Ulf Menyes, Martin Erhardt, Michael Muller, Martina Pohl, Dorte Rother
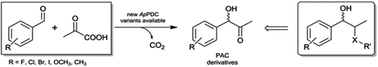
By the combination of biocatalyst design and reaction engineering, the so far not stereoselectively accessible (S)-phenylacetylcarbinol could be enzymatically synthesized with product concentrations >48 g L-1 and an enantiomeric excess up to 97%.
By the combination of biocatalyst design and reaction engineering, the so far not stereoselectively accessible (S)-phenylacetylcarbinol could be enzymatically synthesized with product concentrations >48 g L-1 and an enantiomeric excess up to 97%.
Asymmetric synthesis of (S)-phenylacetylcarbinol – closing a gap in C-C bond formation
Asymmetric synthesis of (S)-phenylacetylcarbinol – closing a gap in C–C bond formation
View original post 230 more words
Filed under: Uncategorized