PF-04745637
cas 1917294-46-2
MW 509.00, MF C27 H32 Cl F3 N2 O2
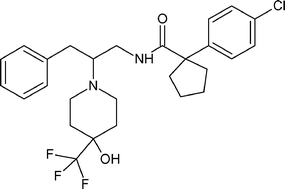
Cyclopentanecarboxamide, 1-(4-chlorophenyl)-N-[2-[4-hydroxy-4-(trifluoromethyl)-1-piperidinyl]-3-phenylpropyl]-
rac-1-(4-Chlorophenyl)-N-f2-r4-hvdroxy-4-(trifluoromethyl)piperidin-1-vn-3-phenylpropyDcyclopentanecarboxamide
|
PRODUCT PATENT WO-2016067143-A1
|
| Applicants: | PFIZER INC. [US/US]; 235 East 42nd Street New York, New York 10017 (US) |
| Inventors: | SWAIN, Nigel Alan; (GB). PRYDE, David Cameron; (GB). RAWSON, David James; (GB). RYCKMANS, Thomas; (GB). SKERRATT, Sarah Elizabeth; (GB). AMATO, George Salvatore; (US). MARRON, Brian Edward; (US). REISTER, Steven Michael; (US). |

TrpA1 is a member of the Transient Receptor Potential (Trp) family of ion channels. It was first described as being activated in response to noxious cold. It is activated by a number of exogenous chemical compounds and some endogenous inflammatory mediators. It has also been reported to be activated in response to mechanical stress.
There is substantial evidence for the involvement of TrpA1 in the physiology of pain, including neuropathic and inflammatory pain, and in pruritus (itch). For example, see:
Bautista, D.M. et al., “TRPA 1: A Gatekeeper for Inflammation” , Annu. Rev. Physiol.2013, 75, 181-200;
Bishnoi, M. & Premkumar, L.S., “Changes in TRP Channels Expression in Painful
Conditions”, Open Pain Journal 2013, 6(Suppl. 1), 10-22;Brederson, J.-D. et al., “Targeting TRP channels for pain relief, Eur. J. Pharmacol.2013, 716, 61-76;
Radresa, O. et al., “Roles of TRPAI in Pain Pathophysiology and Implications for the Development of a New Class of Analgesic Drugs”, Open Pain Journal 2013, 6(Suppl. 1), 137-153; and Toth, B.I. & Biro, T., “TRP Channels and Pruritus” , Open Pain Journal 2013, 6(Suppl.1), 62-80.
There is a continuing interest in finding new compounds that interact with TrpA1.
![Image result for SWAIN, Nigel Alan]() Nigel Swain
Nigel Swain
PATENT
E8 that is 1-(4-chlorophenyl)-/V-[2-(4-hydroxy-4-(trifluoromethyl)piperidin-1-yl)-3-phenylpropyl]-cyclopentanecarboxamide, or a pharmaceutically acceptable salt thereof. This compound is represented by formula (lE).

Example 1
rac-1-(4-Chlorophenyl)-N-f2-r4-hvdroxy-4-(trifluoromethyl)piperidin-1-vn-3-phenylpropyDcyclopentanecarboxamide

Method 1
To a solution of rac-1-(1-amino-3-phenylpropan-2-yl)-4-(trifluoromethyl)piperidin-4-ol (Preparation 2, 50 mg, 0.214 mmol) in DMF (1 mL) was added 1-(4-chlorophenyl)cyclopentanecarboxylic acid (37 mg, 0.165 mmol), DIPEA (0.035 mL, 0.198 mmol) and EDCI (38 mg, 0.198 mmol), followed by HOBt (30 mg, 0.198 mmol) and the reaction was stirred at room temperature for 18 hours. Water was added and the reaction stirred for a further 2 hours. DCM was added with further stirring for 1 hour followed by elution through a phase separation cartridge. The organic filtrate was concentrated in vacuo. The residue was dissolved in MeOH and treated with ethereal HCI with standing for 18 hours. The resulting suspension was filtered and triturated with EtOAc, heptanes and TBME to afford the title compound as the hydrochloride salt (69 mg, 82%).
1H NMR (400MHz, DMSO-d6): δ ppm 1.50-1.60 (m, 4H), 1.70-1.90 (m, 4H), 2.15-2.25 (m, 2H), 2.40-2.48 (m, 2H), 2.70-2.80 (m, 1 H), 3.05-3.25 (m, 6H), 3.47-3.62 (m, 2H), 6.38 (br s, 1 H), 7.20-7.40 (m, 9H), 7.80 (br m, 1 H).
MS m/z 509 [M+H]+
Example 1 may also be prepared according to the following method:
A mixture of 1-(4-chlorophenyl)cyclopentanecarboxylic acid (25.7 g, 114 mmol), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium-3-oxid-hexafluoro phosphate (49.4 g, 130 mmol) and N,N-diisopropylethylamine (40 mL, 229 mmol) in DMF (475 mL) was stirred at room temperature for 15 minutes. To this mixture was added a solution of 1-(1-amino-3-phenylpropan-2-yl)-4-(trifluoromethyl)piperidin-4-ol (Preparation 2, 31.4 g, 104 mmol) in DMF (200 mL). The reaction was stirred at room temperature for 18 hours before partitioning between EtOAc (600 mL) and saturated aqueous sodium hydrogen carbonatesolution (600 mL). The aqueous layer was washed with EtOAc (2 x 600 mL). The combined organic layers were washed with water (600 mL), brine (600 mL), dried over sodium sulphate and concentrated in vacuo. The residue was purified using silica gel column chromatography eluting with 0: 1 to 1 : 1 EtOAc: heptanes to afford the title compound (44 g, 76%).
1H NMR (400MHz, CDCI3): δ ppm 1.35 (br s, 1 H), 1.49-1.85 (m, 6H), 1.90-1.99 (m, 2H), 2.25-2.55 (m, 7H), 2.56-2.70 (m, 1 H), 2.75-3.00 (m, 4H), 3.23-3.31 (m, 1 H), 5.87 (br s, 1 H), 7.07 (d, 2H), 7.16-7.30 (m, 7H).
MS m/z 509 [M+H]+
Examples 2 and 3
IS) and (R)-1-(4-Chlorophenyl)-N-f2-r4-hvdroxy-4-(trifluoromethyl)piperidin-1-vn-3-phenylpropyl)cyclopentanecarboxamide

Example 2
To a suspension of (S)-1-(1-amino-3-phenylpropan-2-yl)-4-(trifluoromethyl)piperidin-4-ol (Preparation 3, 70 mg, 0.232 mmol) and 1-(4-chlorophenyl)cyclopentanecarboxylic acid (57.3 mg, 0.255 mmol) in acetonitrile (0.8 mL) was added triethylamine (0.133 mL, 0.928 mmol) followed bypropylphosphonic anhydride (50% wt solution in EtOAc, 0.21 mL, 0.35 mmol). The reaction was stirred at room temperature for 1.5 hours after which the solution was purified directly by silica gel column chromatography eluting with 0-30% EtOAc in heptanes to afford the title compound (75 mg, 64%).
[a]D20 = +9.6 in DCM [20 mg/mL]
ee determination:
Column: ChiralTech AD-H, 250×4.6 mm, 5 micron.
Mobile phase A: CO2; Mobile phase B: MeOH with 0.2% ammonium hydroxide Gradient: 5% B at 0.00 mins, 60% B at 9.00 mins; hold to 9.5 mins and return to 5% B at 10 mins. Flow rate 3 mL/min.
Rt = 5.047 minutes, ee = 95%
Example 2 may also be prepared from rac-1-(4-chlorophenyl)-N-{2-[4-hydroxy-4- (trifluoromethyl)piperidin-1-yl]-3-phenylpropyl}cyclopentanecarboxamide(Example 1).
The racemate was separated into two enantiomers using preparative chiral chromatography as described below:
Chiralpak IA, 4.6x250mm, 5 micron.
Mobile phase: Hexane:DCM:EtOH:DEA 90:8:2:0.1
Flow rate: 1 mL/min
Rt = 8.351 minutes and Rt = 10.068 minutes
The first eluting isomer is Example 2: (S)-1-(4-chlorophenyl)-N-{2-[4-hydroxy-4-(trifluoromethyl)piperidin-1-yl]-3-phenylpropyl}cyclopentanecarboxamide. ee = 100% The second eluting isomer is Example 3: (R)-1-(4-chlorophenyl)-N-{2-[4-hydroxy-4-(trifluoromethyl)piperidin-1-yl]-3-phenylpropyl}cyclopentanecarboxamide. ee = 99.62% The compound of Example 2 prepared from the chiral separation method is identical by a-rotation and retention time to the compound of Example 2 prepared as the single enantiomer described above.
MS m/z 509 [M+H]+
1H NMR (400MHz, DMSO-d6): δ 1.30-1.80 (m, 10H), 2.20-2.30 (m, 1 H), 2.35-2.60 (m, 6H), 2.65-2.85 (m, 4H), 3.00-3.15 (m, 1 H), 5.50 (br s, 1 H), 6.95-7.00 (m, 1 H), 7.05-7.15 (m, 2H), 7.20-7.35 (m, 6H) ppm
PAPER
The discovery of a potent series of carboxamide TRPA1 antagonists
DOI: 10.1039/C6MD00387G, http://pubs.rsc.org/en/Content/ArticleLanding/2016/MD/C6MD00387G?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FMD+%28RSC+-+Med.+Chem.+Commun.+latest+articles%29#!divAbstract
. Please note PF-6667294 is Compound 4 and PF-4746537 is Compound 8.
A series of potent and selective carboxamide TRPA1 antagonists were identified by a high throughput screen. Structure–activity relationship studies around this series are described, resulting in a highly potent example of the series. Pharmacokinetic and skin flux data are presented for this compound. Efficacy was observed in a topical cinnamaldehyde flare study, providing a topical proof of pharmacology for this mechanism. These data suggest TRPA1 antagonism could be a viable mechanism to treat topical conditions such as atopic dermatitis.



Discovery and development of TRPV1 antagonists
https://en.wikipedia.org/wiki/Discovery_and_development_of_TRPV1_antagonists
/////////////PF-04745637, PF 04745637, PF04745637, PFIZER, PRECLINICAL, TRPV1 antagonists, atopic dermatitis, 1917294-46-2
c1(ccccc1)CC(CNC(=O)C3(c2ccc(cc2)Cl)CCCC3)N4CCC(CC4)(O)C(F)(F)F
Filed under: Preclinical drugs, Uncategorized Tagged: 1917294-46-2, PF-04745637, PF04745637



