
Continuous Flow Stereoselective Synthesis of (S)-Warfarin
The same catalytic packed-bed reactor was used for the preparation of (S)-warfarin 107 under continuous flow conditions (Scheme ).A solution of 4-OH-coumarin 104, benzalacetone105, and trifluoroacetic acid as a cocatalyst in dioxane was flowed into the reactor containing the polystyrene-supported 9-amino-epi-quinine 122. With a residence time of 5 h at 50 °C, we were able to isolate the product in up to 90% yield and up to 87% ee. Further studies are needed in order to optimize the reaction under continuous flow conditions; however, the proposed protocol already offers the possibility to extend catalyst’s lifetime, longer than in batch mode, further suggesting interesting future applications for the catalytic reactors.
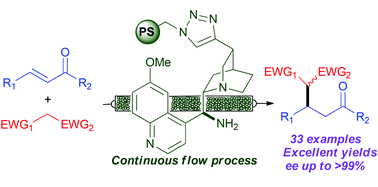
The Pericàs group published the stereoselective Michael addition of ethyl nitroacetate to benzalacetone promoted by polystyrene-supported 9-amino-9-deoxy-epi-quinine 126 under continuous flow conditions. It should be pointed out that the polystyrene in our hands is a highly reticulated, insoluble polymer, while the polystyrene used by the Pericàs group is a swelling resin; a careful choice of the reaction solvent should be done, as this may affect the reaction course. The functionalized resin was packed into a Teflon tube between two plugs of glass wool. The reaction was run by pumping a solution of the two reagents and benzoic acid as a cocatalyst in CHCl3 (chosen after careful solvent screening) at 30 °C for 40 min residence time. Notably, 3.6 g (12.9 mmol) of the desired adducts were collected in 21 h of operation in roughly 1/1 dr and 97/98% ee.
Porta, R.; Benaglia, M.; Puglisi, A. Unpublished results.
Izquierdo, J.; Ayats, C.; Henseler, A. H.; Pericàs, M. A. Org. Biomol. Chem. 2015, 13, 4204, DOI: 10.1039/C5OB00325C

A polystyrene-supported 9-amino(9-deoxy)epi quinine derivative for continuous flow asymmetric Michael reactions
E-mail: mapericas@iciq.es
Fax: +34 977920244
Tel: +34 977920243
DOI: 10.1039/C5OB00325C
A polystyrene (PS)-supported 9-amino(9-deoxy)epi quinine derivative catalyzes Michael reactions affording excellent levels of conversion and enantioselectivity using different nucleophiles and structurally diverse enones. The highly recyclable, immobilized catalyst has been used to implement a single-pass, continuous flow process (residence time: 40 min) that can be operated for 21 hours without significant decrease in conversion and with improved enantioselectivity with respect to batch operation. The flow process has also been used for the sequential preparation of a small library of enantioenriched Michael adducts.

Synthesis:
There are 3 types of Warfarin:
1. Racemic Warfarin
2. S-Warfarin
3. R-Warfarin
As there are different types different synthetic routes are required. Firstly, looking at the racemic Warfarin followed by the asymetric Warfarin (S- and R- Warfarin).
The usual synthetic route for racemic Warfarin involves a base/acid catalysed Michael condensation reaction of 4-hydroxycoumarin with benzalacetone. These reactants are either refluxed in water for approximately 4-8 hours or refluxed with pyridine which gives a saturated yield. The mechanism is shown below:
The yield when this reaction is reflux with water is 48%.
During recent years it has been found that one of the possible enantiomers usually has a pharmacological profile that is superior to the racemate. Hence pharmaceutical companies have been replacing exisiting racemic drugs with their pure enantiomeric form.
In the case of Warfarin it was found that S-Warfarin is the superior enantiomer being 6 times more active than R-Warfarin. There are 2 main methods to form a pure enantiomeric form of Warfarin.
1. Asymmetric hydrogenation: This was developed by DuPont Merk Pharmaceutical. It involves the a DuPHOS-Rh(I) catalysed hydrogenation of racemic Warfarin to give the desired enantiomer. Below is the reaction scheme for this synthesis:

This exclusive product is then used in the rest of the synthesis. First reacting it with NaOH to form the sodium salt of the product:

This, then, depending on the enantiomer that is desired, the sodium salt is hydrogenated using either (R,R)-Et-DuPHOS-Rh(I) or (S,S)-Et-DuPHOS-Rh(I) to give S-Warfarin and R-Warfarin respectively:

This route gives enantioselectivities of 82-86% e.e in methanol and 88% e.e in 3:2 isopropanol-methanol. Acidification and a single recrystallisation of the crude product gave R- and S- Warfarin in >98% e.e.
2. Hetero-Diels-Alder cycloaddition: This method was developed in 2001 and the key feature is that it does not use racemic Warfarin as a starting material. Instead it involves a hetero-Diels-Alder cycloaddition of a iso-propenyl ether to 4-hydroxycoumarin (via the use of dry dioxane and a Tietze Base with 5A Molecular sieves at a temperature of 80ºC):
Here S-Warfarin has been synthesised with an e.e of 95%.




General Data:
| Chemical Names: |
|
| Formula: |
C19H16O4 |
| CAS Number: |
81-81-2
|
| Molecular Weight: |
308.33
|
| Structure: |
 |
| Isomers: |
Optical Isomers: S-Warfarin and R-Warfarin
|
| Melting Point /ºC : |
161
|
| Optical Rotation: |
S-Warfarin : -25.5 ± 1º
R-Warfarin : +24.8 ± 1º |

Tautomerization of warfarin substructures, whose combination generates 40 distinct tautomeric forms of warfarin

13 C NMR spectrum (A) and 1 H NMR spectrum (B) of warfarin. Arrows indicate peaks from the open-chain form of warfarin though the intensity is very low. See Figure 1 for numbering of the C atoms. H1(R) and H1(S) are connected to C15; H2 and H3 are connected to C13; and H4 is bonded to C3.
| (R)-(+)-Warfarin |
The structure of Warfarin
Warfarin is optically active, and from the time of it’s discovery it was recognised that the two enantiomers were clinically different in their effect as a drug. So establishing the absolute configuration of the two isomers was a priority.
| R-Warfarin
|
S-Warfarin
|
Hemiketal Ring Formation
| RR-Warfarin
|
SS-Warfarin
|
| RS-Warfarin
|
SR-Warfarin
|
The stereochemical assignment of (−)-(S)-warfarin was initially achieved by relating it to (−)-(R)-beta-phenylcaproic acid through a series of reactions not involving the asymmetric center {B.D.West, S.Preis, C.H.Schroder, & K.P.Link, J.Amer.Chem.Soc.,1961,83, 2676}. This assignment was confirmed by a determination of the crystal and molecular structure, and using the anomalous scattering of oxygen, and absolute configuration of (−)-(S)-Warfarin was measured {E.J.Valente, W.F.Trager and L.H.Jensen, Acta Cryst. 1975. B31, 954}.
The Hemiketal
The primary feature of the structure of (−)-warfarin is the hemiketal ring formed by cyclization of the side-chain keto function and the phenolic hydroxyl in the 4 position of the coumarin ring system. The crystal structure of racemic warfarin has the same feature. In solution n.m.r. spectra shows that the hemiketal is present in acetone solution.
Bond Lengths
The hemiketal bonding is rather weak. Thus the bond lengths within the hemiketal show that the atoms retain some of the characteristic of an open side chain keto group.
The Absolute Configuration
In the open chain keto form warfarin has two isomers, R andS, however the hemiketal introduces a second assymmetric center, so that we can have RR,SS, RS, and SR forms. The crystal structure determination favoured the SS enantiomer in the crystal studied.
Enantiomers & Biochemical Function
The S-isomer is very much more potent than the R isomer in both rats and humans.The S-isomer is stereoselectively oxidized to the inactive 7-hydroxywarfarin, and the keto-group of the R-isomer is stereospecifically reduced to the slightly active R,S-alcohol. Both isomers are oxidized to the inactive 6-hydroxywarfarin.
It is evident that we are dealing with a very complex system indeed; the presence of the hemiketal adds four more enantiomers to the complexity pot. Recent work has unravelled some more of the mechanisms behind the Vitamin K1 antagonism of Warfarin.
Preparation of Coumarins: the Pechmann Condensation
In 1883 Hans von Pechmann and Carl Duisberg {H. v Pechmann, and C. Duisberg, Ber., 1883, 16, 2119} found that phenols condense with beta-ketonic esters in the presence of sulphuric acid, giving coumarin derivatives.

With R1=OH we have 4-hydroxycoumarin, the starting material for the preparation of Warfarin
The reaction is also catalysed by the presence of a Lewis acid such aluminium(III) chloride or other strong Brönstedt acids such as methanesulphonic acid to form a coumarin. The acid catalyses trans-esterification as well as keto-enol tautomerisation.
Bismuth(III) chloride, also a Pechmann catalyst, provides a recent procedure for 4-substituted coumarins.{ An Efficient and Practical Procedure for the Synthesis of 4-Substituted Coumarins Surya K. De*, Richard A. Gibbs, Synthesis, 2005, 1231.}
In another Pechmann condensation synthesis, the ionic liquid 1-butyl-3-methylimidazolium chloroaluminate ([bmim]Cl.2AlCl3) plays the dual role of solvent and Lewis acid catalyst for the reaction of phenols with ethyl acetoacetate leading to coumarin derivatives. Here, the reaction time is reduced drastically even at ambient conditions. {M. K. Potdar, S. S. Mohile, M. M. Salunkhe, Tetrahedron Lett., 2001, 42, 9285}
Solid acid catalysts with the H+ attached to the polymer surface such as Nafion 417 or Amberlyst IR120 can be used. Thus resorcinol reacts with ethyl acetoacetate in boiling toluene in the presence of Nafion sheet to form the coumarin 7-hydroxy-4-methylcoumarin. This preparation forms the basis of a student organic chemistry experiment at Penn State University. In this case the coumarin, {also named, 7-hydroxy-4-methyl-2H-benzo[b]-pyran-2-one} is not a blood thinner but is a drug used in bile therapy, Hymecromone. The material is also, in highly purified form a laser dye, and the starting material for some insecticides!
The Preparation of Warfarin
 Reaction of 4-hydroxycoumarin with benzylacetone underMichael reactionconditions gives racaemic warfarin.
Reaction of 4-hydroxycoumarin with benzylacetone underMichael reactionconditions gives racaemic warfarin.

Imidazolidinone compounds – MacMillan organocatalysts – enable a stereoselective preparation for this reaction
There has been a recent flurry of interest in such assymetric preparation, well cataloged byWikipedia, references 17 to 22. The last reference even puts the stereoselective preparation into the second year undergraduate chemistry laboratory as an innovative ‘green chemistry’ experiment:
The Biochemistry of Warfarin Action
This is a complex biochemical and medical subject, certainly beyond the simple chemistry required for a molecule of the month! Warfarin acts as a Vitamin K antagonist, that is it blocks the action of vitamin K epoxide reductase.
Vitamins K1 and K2

This vitamin is found in brassicas, spinach, parsley, and other green vegetables, avocado pairs are also rich in Vitamin K1.

For Vitamin K2, n signifies a number of five-carbon side chain units, hence MK-n, and except for MK4, is synthesised by gut bacteria. Both vitamins are fat soluble, the “K” deriving from the German “koagulation”. German researchers discovered the K vitamins, and that they are involved in blood clotting.
Vitamin K Cycle
 Vitamin K is a cofactor in the synthesis of blood clotting factors II, VII, IX and X*, this step occurs in the liver and involves the gammacarboxylation of the first 10 glutamic acid residues in the amino-terminal region of the prothrombin clotting factor to generategamma-carboxyglutamate. The gamma-carboxyglutamatee amino acid groups can chelate Ca2+ better than ten replaced glutamate residues, thus providing binding sites for four Vitamin Ks onto the phospholipid membrane during coagulation. The clotting occurs via a cascade*, a kind of biochemical chain reaction. {See Biochemistry by Stryer for the terminology}
Vitamin K is a cofactor in the synthesis of blood clotting factors II, VII, IX and X*, this step occurs in the liver and involves the gammacarboxylation of the first 10 glutamic acid residues in the amino-terminal region of the prothrombin clotting factor to generategamma-carboxyglutamate. The gamma-carboxyglutamatee amino acid groups can chelate Ca2+ better than ten replaced glutamate residues, thus providing binding sites for four Vitamin Ks onto the phospholipid membrane during coagulation. The clotting occurs via a cascade*, a kind of biochemical chain reaction. {See Biochemistry by Stryer for the terminology}
 To work, the Vitamin K must be reduced to its quinol or hydroquinone form. This is achieved with Vitamin K Oxide reductase, which is the step inhibited by S-warfarin, being some three times more potent than R-warfarin. S-warfarin is metabolized primarily by the CYP2C9 enzyme of the cytochrome P450 system. The R-warfarin is metabolized by the two cytochrome P450 enzymes, CP1A4Y and CYP3A4. Warfarin is very soluble in water, and is absorbed into the blood stream within 90 minutes of taking the pills.
To work, the Vitamin K must be reduced to its quinol or hydroquinone form. This is achieved with Vitamin K Oxide reductase, which is the step inhibited by S-warfarin, being some three times more potent than R-warfarin. S-warfarin is metabolized primarily by the CYP2C9 enzyme of the cytochrome P450 system. The R-warfarin is metabolized by the two cytochrome P450 enzymes, CP1A4Y and CYP3A4. Warfarin is very soluble in water, and is absorbed into the blood stream within 90 minutes of taking the pills.
So far as the enantiomers are concerned, racaemic warfarin has a half life of around 40 hours, the two enantiomers, having half lives: R-warfarin, 45 hours; S-warfarin, 29 hours.
During my review for MoTM, necessarily hurried, I have not been able to find out if the hemiketal, with the four enantiomers is involved. That the hemiketal is weak is shown by the crystal structure study, so, in any case these enantiomers will have short half lives. It all adds to the complexity.
An application of an asymmetric synthesis with a DuPhos ligand is the hydrogenation of dehydrowarfarin to warfarin:[9]
The first practical asymmetric synthesis of R and S-Warfarin Andrea Robinson and Hui-Yin Li John Feaster Tetrahedron Letters Volume 37, Issue 46, 11 November 1996, Pages 8321-8324doi:10.1016/0040-4039(96)01796-0
Links & References
- Biochemistry, Lubert Stryer, Freeman and Co. 1981; the basics of blood clotting are described in Chapter 8.
- The Crystal and Molecular Structure and Absolute Configuration of (−)(S)-Warfarin, E.J.Valente, W.F.Trager and L.H.Jensen, Acta Cryst. 1975. B31, 954. A seminal paper on the structure of S-warfarin
-
- Wikipedia provides an invaluable source of knowledge, and better still, of references!
- Warfarin
- Hans von Pechmann
- Michael reaction
- The Pechmann Condensation Mechanism
- Rodenticides
- Thrombin – Prothrombin
- Organocatalytic Asymmetric Michael Reaction of Cyclic 1,3-Dicarbonyl Compounds and Unsaturated Ketones – A Highly Atom-Economic Catalytic One-Step Formation of Optically Active Warfarin Anticoagulant, N.Halland, T.Hansen and K.A.Jørgensen, Angew. Chem. Int. Ed. 2003, 42(40), 4955.
- Studies on 4-Hydroxycoumarins. V. The Condensation of alpha,beta-Unsaturated Ketones with 4-Hydroxycoumarin. M. Ikawa, M.A. Stahmann and K.P.Link, J.Amer.Chem.Soc 1944, 66, 902.
- Pharmacology and Management of the Vitamin K Antagonists, an excellent and freely downloadable, CHEST article from a group of doctors and pharmacologists.
- Vitamin K: paper for students
- Vitamin K: Linus Pauling Institute article.
- Warfarin by Yunas Bhonoah of Imperial College. A student project. The crystal structure paper was not found, nor the differing effects of the two enantiomers. However see the section on themechanism of action of Warfarin
- Pharmacogenetics of warfarin elimination and its clinical implications. A paper dealing with pharmacogenetic polymorphism of cytochrome P450
//////////////////////Continuous Flow, Stereoselective Synthesis, (S)-Warfarin, FLOW CHEMISTRY, FLOW SYNTHESIS
Filed under: flow synthesis Tagged: (S)-Warfarin, continuous flow, flow chemistry, FLOW SYNTHESIS, Stereoselective Synthesis











