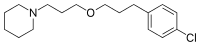
Pitolisant
1-(3-(3-(4-Chlorophenyl)propoxy)propyl)piperidine
MF C17H26ClNO
MW 295.1703
(Wakix®)Approved EU 31/3/2016, Narcolepsy
A histamine H3 receptor antagonist/inverse agonist used to treat narcolepsy.

BF-2649; BF-2.649; FUB-649, Ciproxidine, Tiprolisant
CAS 362665-56-3, 362665-57-4 (oxalate)
CAS 903576-44-3(Pitolisant Hydrochloride)
1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine hydrochloride (1:1)
| Molecular Weight | 332.31 |
| Formula | C17H26ClNO ● HCl |

Bioprojet INNOVATOR
Jean-Charles Schwartz, Jeanne-Marie Lecomte
Pitolisant (INN) or tiprolisant (USAN) is a histamine receptor inverse agonist/antagonist selective for the H3 subtype.[1] It hasstimulant and nootropic effects in animal studies,[2] and may have several medical applications, having been researched for the treatment of narcolepsy, for which it has been granted orphan drug status in the EU and US.[3][4] It is currently in clinical trials forschizophrenia and Parkinson’s disease.[4][5][6]
Pitolisant hydrochloride was approved by European Medicine Agency (EMA) on Mar 31, 2016. It was developed and marketed as Wakix® by Bioprojet in EU.

Pitolisant hydrochloride is an antagonist/inverse agonist of the histamine H3 receptor, which is indicated in adults for the treatment of narcolepsy with or without cataplexy.
Wakix® is available as tablet for oral use, containing 4.5 mg and 18 mg of Pitolisant hydrochloride. The initial dose of 9 mg (two 4.5 mg, tablets) per day, and it should be used at the lowest effective dose, depending on individual patient response and tolerance, according to an up-titration scheme, without exceeding the dose of 36 mg/day.
Pitolisant was developed by Jean-Charles Schwartz, Walter Schunack and colleagues after the former discovered H3 receptors.[7]Pitolisant was the first clinically used H3 receptor inverse agonist.
Pitolisant, also known as Tiprolisant, is a histamine receptor inverse agonist/antagonist selective for the H3 subtype. It has stimulant and nootropic effects in animal studies, and may have several medical applications, having been researched for the treatment of narcolepsy, for which it has been granted orphan drug status in the EU and US. It is currently in clinical trials for schizophrenia and Parkinson’s disease. Pitolisant was the first clinically used H3 receptor inverse agonist.

The European Medicines Agency (EMA) has recommended granting marketing authorization for pitolisant (Wakix, Bioprojet Pharma) for narcolepsy with or without cataplexy, the agency announced today.
Narcolepsy is a rare sleep disorder that affects the brain’s ability to regulate the normal sleep-wake cycle, leading to excessive daytime sleepiness, including the sudden urge to sleep, and disturbed night-time sleep. Some patients also experience sudden episodes of cataplexy, potentially causing dangerous falls and increasing the risks for accidents, including car accidents. Symptoms of narcolepsy can be severe and significantly reduce quality of life.
Pitolisant “will add to the available treatment options for narcolepsy. It is a first-in-class medicine that acts on histamine H3 receptors in the brain. This leads to increased histamine release in the brain, thereby enhancing wakefulness and alertness,” the EMA notes in a news release.
The EMA recommendation for approval of pitolisant is based on an evaluation of all available safety and efficacy data conducted by the Committee for Medicinal Products for Human Use (CHMP). The data include two pivotal placebo-controlled trials involving 259 patients, as well as one uncontrolled, open-label study involving 102 patients with narcolepsy and one supportive study in 105 patients.
The studies showed that pitolisant was effective in reducing excessive daytime sleepiness in patients with narcolepsy. The beneficial effect of the drug on cataplexy was demonstrated in one of the pivotal studies as well as in the supportive study.
No major safety concerns with pitolisant emerged in testing. Insomnia, headache, and nausea were among the most common adverse effects observed in the clinical trials, and the CHMP decided on measures to mitigate these risks, the EMA said. The CHMP also requested the company conduct a long-term safety study to further investigate the safety of the drug when used over long periods.
Pitolisant for narcolepsy received orphan designation from the Committee for Orphan Medicinal Products in 2007. Orphan designation provides medicine developers access to incentives, such as fee reductions for scientific advice, with the aim of encouraging the development of treatments for rare disorders.
The CHMP opinion will now be sent to the European Commission for the adoption of a decision on a European Union–wide marketing authorization. Once that has been granted, each member state will decide on price and reimbursement based on the potential role/use of this medicine in the context of its national health system.

Narcolepsy-cataplexy.
Narcolepsy-cataplexy, or Gelineau syndrome, is a rare but serious disorder characterized by excessive daytime sleepiness which can be an extreme hindrance to normal professional and social activities, and which is accompanied by more or less frequent attacks of cataplexy (a sudden loss of muscle tone triggered by emotions as varied as laughter or fear) and erratic episodes of REM sleep (during wakefulness and during sleep), sometimes associated with hypnagogic hallucinations. Moreover, individuals with narcolepsy have various degrees of cognitive impairment and tend to be obese (reviewed by Dauvilliers et al., Clin. Neurophysiol., 2003, 114, 2000; Baumann and Bassetti, Sleep Med. Rev., 2005, 9, 253).
The disorder is caused by the loss of a group of neurons in the brain which produce two peptides, orexins, also known as hypocretins, located in the anterior hypothalamus and projecting to the main groups of aminergic neurons which regulate wakefulness and sleep. Patients with the disorder generally have very low levels of orexins in cerebrospinal fluid. Orexin knock-out mice display many of the symptoms seen in narcoleptic subjects, confirming the role of these peptides and thereby providing an excellent animal model of the disease (Chemelli et al., Cell, 1999, 98, 437).
Several types of treatments which can improve the symptoms of narcolepsy already exist, although they do not completely relieve symptoms and, furthermore, can cause significant side effects limiting their usefulness.
For instance, amphetamines or analogues such as methylphenidate which release catecholamines are used to treated daytime sleepiness, but these agents induce a state of excessive excitation as well as cardiovascular disturbances and also carry a potential for drug addiction.
Modafinil, a drug whose mechanism of action is unclear, also improves daytime sleepiness without causing as many side effects as amphetamines. Nonetheless, its efficacy is limited and it can cause headaches and nausea, particularly at high doses. Moreover amphetamines and/or modafinil do not appear to improve some of the most disabling symptoms of the disease, particularly cataplexy attacks, cognitive deficits and weight gain. With regard to cataplexy, treatments include antidepressants and oxybate. Effectiveness of the former has not been demonstrated (Cochrane Database Syst. Rev., 2005, 20, 3), and the latter is a drug of illegal abuse and its use is restricted.
It has also been shown that histamine H3 receptor antagonists induce the activation of histaminergic neurons in the brain which release histamine, a neurotransmitter with a crucial role in maintaining wakefulness (Schwartz et al., Physiol. Rev. 1991, 71, 1).
PATENT
Pharmaceutical products with histamine H3 receptor ligand properties and 0 subsequent pharmacological activities thereof are described in EP-980300. An especially important product among those disclosed is 1-[3-[3-(4- chlorophenyl)propoxy] propyl]-piperidine. This compound is disclosed as the free base and as the oxalate salt.
5 The use of 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine as the free base is limited because of its oily nature. On the contrary, 1-[3-[3-(4- chlorophenyl)propoxy]propyl]-piperidine oxalate is a crystalline substance but its low aqueous solubility (0.025 g/ml at 230C) also limits its use as a
pharmaceutical ingredient.
0
Subsequent patents EP-1100503 and EP-1428820 mention certain salts of 1- [3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine. However, the only one specifically described is the oxalate salt. The crystalline monohydrochloride salt is not described.
Example 1 : 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine
According to the method disclosed in EP-982300, Example 78, sodium 3-piperidinopropanolate (2.127 kg; 12.88 mol), 3-(4-chlorophenyl)propyl mesylate (1.121 kg; 4.51 mol) and 0.322 mol of 15-crown-5 in 4.5 kg of dry toluene were refluxed for 4 hours. The solvent was evaporated and the residue purified by column chromatography on silica gel (eluent: methylene chloride/methanol (90/10)). The obtained oil was distilled in a fractionating equipment at reduced pressure (0.3-0.7 mmHg) and with a heating jacket at 207-2100C. The head fractions and the distilled fraction at 0.001-0.010 mmHg with a jacket temperature of 180-2000C were collected. The obtained oil (1.0 kg; 3.38 mol) corresponds to 1-[3-[3-(4-chlorophenyl)propoxy] propyl]-piperidine. Yield 75%.
Example 2: 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine
monohydrochloride
Preparation
Distilled 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine (1.0 kg) and anhydrous ethyl acetate (4.5 kg) are transferred to a 10-L glass vessel fitted with a cooling bath and a gas inlet. A stream of gaseous hydrogen chloride is bubbled in the reaction mixture at 20-250C.
The pH of the solution is checked by taking a 0.5 mL sample of the reaction mixture and diluting it with 5 mL of deionized water. The final pH must be about 3-4.
The mixture is cooled to -10°C-(-12°C) and stirred at this temperature for 1 h. The precipitate is filtered by using a sintered glass filter and washed with 0.5 L of anhydrous ethyl acetate previously cooled to 0-50C. The product is dried in a vacuum oven at 5O0C for a minimum period of 12 hours. The resulting crude 1 -[3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine monohydrochloride weighs 1.10 kg.
Purification
A mixture of the above-described crude, 3.98 kg of anhydrous ethyl acetate and 0.35 kg of /-propanol is heated slowly at 55-6O0C in a 10-L glass vessel fitted with a heating and cooling system. When the solution has been completed, it is filtered through a heat-isolated sintered glass filter, keeping the temperature at 55-6O0C. The solution is transferred to a 10 L glass vessel and the mass is slowly cooled to 0-50C for about 1 hour. The mixture is stirred at this temperature for 1 hour and the precipitate is filtered through a sintered glass filter. The solid is washed with a mixture of 1.6 kg of anhydrous ethyl acetate and 0.14 kg of /-propanol cooled at 0-50C. The solid is dried in a vacuum oven at 5O0C for a minimum period of 12 hours. M. p. 117-1190C. Yield 80%.
IR spectrum (KBr): bands at 1112 and 1101 (C-O Ether/ St. asym), 2936 and 2868 (Alkane CH(CH2)) / St.), 1455 (Alkane CH(CH2)) / Deform.), 2647 and 2551 (Amine Salt / St.), 1492 (Amine / St.), 802 (Aromatic / Deform.) cm“1.
SEE
Eur. J. Pharm. Sci. 2001, 13, 249–259.
References
- Celanire S, Wijtmans M, Talaga P, Leurs R, de Esch IJ (December 2005). “Keynote review: histamine H3 receptor antagonists reach out for the clinic”. Drug Discov. Today. 10 (23-24): 1613–27. doi:10.1016/S1359-6446(05)03625-1. PMID 16376822.
- Ligneau X, Perrin D, Landais L, Camelin JC, Calmels TP, Berrebi-Bertrand I, Lecomte JM, Parmentier R, Anaclet C, Lin JS, Bertaina-Anglade V, la Rochelle CD, d’Aniello F, Rouleau A, Gbahou F, Arrang JM, Ganellin CR, Stark H, Schunack W, Schwartz JC. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: Preclinical pharmacology. Journal of Pharmacology and Experimental Therapeutics. 2007 Jan;320(1):365-75. PMID 17005916
- Lin JS, Dauvilliers Y, Arnulf I, Bastuji H, Anaclet C, Parmentier R, Kocher L, Yanagisawa M, Lehert P, Ligneau X, Perrin D, Robert P, Roux M, Lecomte JM, Schwartz JC. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiology of Disease. 2008 Apr;30(1):74-83. PMID 18295497
- ^ Jump up to:a b Prous Science: Molecule of the Month September 2011
- Ligneau X, Landais L, Perrin D, Piriou J, Uguen M, Denis E, Robert P, Parmentier R, Anaclet C, Lin JS, Burban A, Arrang JM, Schwartz JC. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochemical Pharmacology. 2007 Apr 15;73(8):1215-24. PMID 17343831
- Stocking EM, Letavic MA (2008). “Histamine H3 antagonists as wake-promoting and pro-cognitive agents”. Current Topics in Medicinal Chemistry. 8 (11): 988–1002. doi:10.2174/156802608784936728. PMID 18673168.
- Schwartz, Jean-Charles (May 2011). “The histamine H3 receptor: from discovery to clinical trials with pitolisant”. BPJ. doi:10.1111/j.1476-5381.2011.01286.x.
REFERENCES
1: Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med. 2014 Jun;15(6):681-7. doi: 10.1016/j.sleep.2014.01.021. Epub 2014 Mar 18. PubMed PMID: 24854887.
2: Dauvilliers Y, Bassetti C, Lammers GJ, Arnulf I, Mayer G, Rodenbeck A, Lehert P, Ding CL, Lecomte JM, Schwartz JC; HARMONY I study group. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013 Nov;12(11):1068-75. doi: 10.1016/S1474-4422(13)70225-4. Epub 2013 Oct 7. PubMed PMID: 24107292.
3: Nirogi R, Ajjala DR, Kandikere V, Pantangi HR, Jonnala MR, Bhyrapuneni G, Muddana NR, Vurimindi H. LC-MS/MS method for the determination of pitolisant: application to rat pharmacokinetic and brain penetration studies. Biomed Chromatogr. 2013 Nov;27(11):1431-7. doi: 10.1002/bmc.2939. Epub 2013 Jun 13. PubMed PMID: 23760876.
4: Kasteleijn-Nolst Trenité D, Parain D, Genton P, Masnou P, Schwartz JC, Hirsch E. Efficacy of the histamine 3 receptor (H3R) antagonist pitolisant (formerly known as tiprolisant; BF2.649) in epilepsy: dose-dependent effects in the human photosensitivity model. Epilepsy Behav. 2013 Jul;28(1):66-70. doi: 10.1016/j.yebeh.2013.03.018. Epub 2013 May 8. PubMed PMID: 23665640.
5: Uguen M, Perrin D, Belliard S, Ligneau X, Beardsley PM, Lecomte JM, Schwartz JC. Preclinical evaluation of the abuse potential of Pitolisant, a histamine H₃ receptor inverse agonist/antagonist compared with Modafinil. Br J Pharmacol. 2013 Jun;169(3):632-44. doi: 10.1111/bph.12149. PubMed PMID: 23472741; PubMed Central PMCID: PMC3682710.
6: Brabant C, Charlier Y, Tirelli E. The histamine H₃-receptor inverse agonist pitolisant improves fear memory in mice. Behav Brain Res. 2013 Apr 15;243:199-204. doi: 10.1016/j.bbr.2012.12.063. Epub 2013 Jan 14. PubMed PMID: 23327739.
7: Zhang DD, Sisignano M, Schuh CD, Sander K, Stark H, Scholich K. Overdose of the histamine H₃ inverse agonist pitolisant increases thermal pain thresholds. Inflamm Res. 2012 Nov;61(11):1283-91. doi: 10.1007/s00011-012-0528-5. Epub 2012 Jul 21. PubMed PMID: 22820944.
8: Inocente C, Arnulf I, Bastuji H, Thibault-Stoll A, Raoux A, Reimão R, Lin JS, Franco P. Pitolisant, an inverse agonist of the histamine H3 receptor: an alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness. Clin Neuropharmacol. 2012 Mar-Apr;35(2):55-60. doi: 10.1097/WNF.0b013e318246879d. PubMed PMID: 22356925.
9: Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011 Jun;163(4):713-21. doi: 10.1111/j.1476-5381.2011.01286.x. Review. PubMed PMID: 21615387; PubMed Central PMCID: PMC3111674.
 |
|
| Names | |
|---|---|
| IUPAC name
1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine
|
|
| Other names
BF2.649
|
|
| Identifiers | |
903576-44-3  |
|
| ChEMBL | ChEMBL462605  |
| ChemSpider | 8123714  |
| Jmol 3D model | Interactive image |
| PubChem | 9948102 |
| Properties | |
| C17H26ClNO | |
| Molar mass | 295.846 g/mol |
| Pharmacology | |
| N07XX11 (WHO) | |
//////////Pitolisant Hydrochloride, Wakix, histamine H3 receptor antagonist/inverse agonist, narcolepsy, orphan drug, tiprolisant
ClC1=CC=C(CCCOCCCN2CCCCC2)C=C1
Filed under: 0rphan drug status, EU 2016 Tagged: EU 2016, histamine H3 receptor antagonist/inverse agonist, narcolepsy, Orphan Drug, Pitolisant, Pitolisant Hydrochloride, tiprolisant, Wakix
