
 5-Chloro-N-{[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinophenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide
5-Chloro-N-{[(5S)-2-oxo-3-[4-(3-oxo-4-morpholinophenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide
Originator and Manufacturer:Bayer
Marketer in the US: Johnson & Johnson
Sales: $1.3 billion (2013)

In September 2008, Health Canada granted marketing authorization for rivaroxaban for the prevention of venous thromboembolism(VTE) in people who have undergone elective total hip replacement or total knee replacement surgery.[8]
In September 2008, the European Commission granted marketing authorization of rivaroxaban for the prevention of venous thromboembolism in adults undergoing elective hip and knee replacement surgery.[9]
On July 1, 2011, the U.S. Food and Drug Administration (FDA) approved rivaroxaban for prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in adults undergoing hip and knee replacement surgery.[5]
On November 4, 2011, the U.S. FDA approved rivaroxaban for stroke prophylaxis in patients with non-valvular atrial fibrillation.

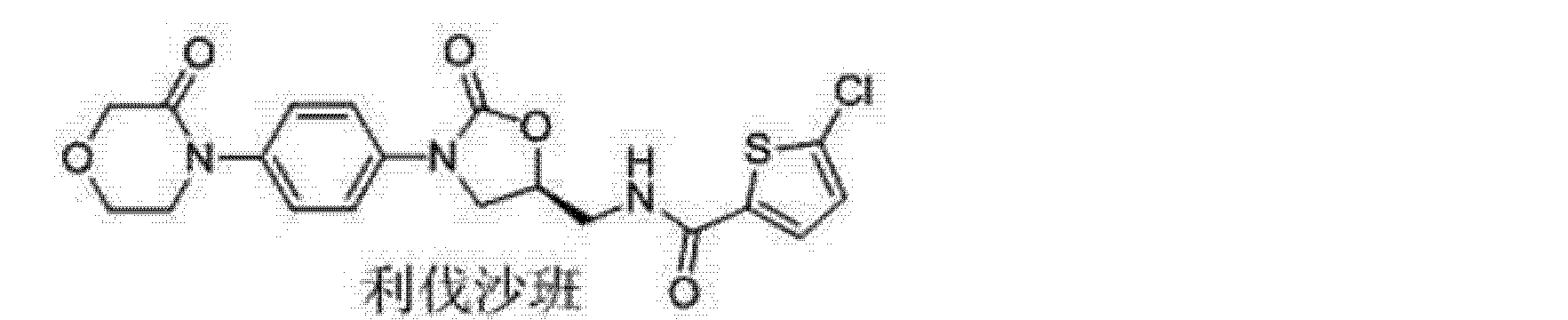
The drug compound having the adopted name “Rivaroxaban” has chemical name, 5-chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-l,3-oxazolidin-5- yljmethyl)-2-thiophenecarboxamide; and has the structural formula I,

Formula I
The commercial pharmaceutical product XARELTO® tablets, contains rivaroxaban as active ingredient. Rivaroxaban is a factor Xa inhibitor useful as oral anticoagulant. Rivaroxaban can be used for the prevention and treatment of various thromboembolic diseases, in particular of deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infract, angina pectoris and restenoses after angioplasty or aortocoronary bypass, cerebral stroke,
transitory ischemic attacks, and peripheral arterial occlusive diseases.
U.S. Patent No. 7, 157,456 describes Rivaroxaban and process for the preparation thereof. The process of US ‘456 for rivaroxaban involves reaction of 2-[(2S)-2-oxiranylmethyl]-lH-isoindole-l,3(2H)-dione with 4-(4-aminophenyl)-3-morpholinone to provide 2-((2R)-2-hydroxy-3- { [4-(3-oxo-4-morpholiny)phenyl]amino Jpropyl)- lH-isoindole- 1 ,3(2H)-dione, which on cyclization using Ν,Ν-carbonyl diimidazole to afford 2-({5S)-2-Oxo-3-[4-(3-oxo-4-morpholiny)phenyl]-l,3-oxazolidin-5-yl}methyl)-lH-isoindole-l,3(2H)-dione, which on reacted with methylamine followed by reaction with 5-chlorothiophene-2-carbonyl chloride to provide Rivaroxaban.
Various processes for the preparation of rivaroxaban, its intermediates, and related compounds are disclosed in U.S. Patent Nos. 7,585,860; 7,351,823, 7,816,355, and 8,101,609; patent application Nos. WO 2011/012321, WO 2012/156983, WO 2012/153155, WO 2013/053739, WO 2013/098833, WO 2013/156936, WO 2013/152168, WO 2013/120464, WO 2013/164833, US 2012/0283434 and US 2013/184457; and J. Med. Chem. 2005, 48, 5900-5908.

PAPER CONTAING SPECTRAL DATA
a College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310032, P. R. China
b Zhejiang Apeloa Medical Technology Co., Ltd, Dongyang 322118, P. R. China
available (R)-epichlorohydrin with 4-(morpholin-3-one)phenyl isocyanate catalysed by MgI2 etherate as the
key step, in 22% overall yield.
Keywords: (R)-epichlorohydrin, isocyanate, MgI2.etherate, rivaroxaban
STRUCTURE
![ChemSpider 2D Image | Rivaroxaban | C19H18ClN3O5S]()
![Figure CN102786516AD00041]()
SIMILARITY
Chemical structures of linezolid (top) and rivaroxaban (bottom). The shared structure is shown in blue.
IH NMR PREDICT
13 C NMR PREDICT



New patent WO-2015104605
Process for preparing rivaroxaban – comprising the reaction of a thioester compound and its salts with 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholine-3-one.
Wockhardt Ltd
The synthesis of (II) via intermediate (I) is described (example 7, page 15)
4-{4-[(5S)-5-(Aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholine-3-one (formula III) is (I) and rivaroxaban is (II) (claim 1, page 16).
The present invention relates to a process for the preparation of Rivaroxaban and its novel intermediates, or pharmaceutically acceptable salts thereof. The present invention provides novel intermediates, which may be useful for the preparation of Rivaroxaban or its pharmaceutically acceptable salts thereof. The process of preparation by using novel intermediate is very simple cost effective and may be employed at commercial scale. The product obtained by using novel intermediate yield the Rivaroxaban of purity 99% or more, when measured by HPLC. The present invention especially relates to a process for the preparation of Rivaroxaban from thioester of formula II, or a pharmaceutically acceptable salt thereof, wherein R is leaving group.
process includes the step of , reacting thioester of formula IIA or pharmaceutically acceptable salt thereof
Formula IIA

with 4-{4-[(5S)-5-(aminomethyl)-2-oxo-l,3-oxazolidin-3-yl]phenyl}morpholine-3-one of formula III,
Formula III

Formula I

EXAMPLE 7: One pot process for Rivaroxaban
The triphenylphosphine (11.5g) and mercaptobenzothiazole disulphide (15.31g) were taken in methylene chloride and reaction mixture was stirred at 28°C -30°C for 1 hr. The 5-chlorothiophene-2-carboxylic acid (7.2g) and triethylamine (3.8 g) were added to the above reaction mixture. The reaction mixture is stirred at 0°C -25 °C for 1 hr. after 1 hr 4-{4-[(5S)-5-(aminomethyl)-2-oxo-l,3-oxazolidin-3-yl]phenyl}morpholine-3-one (lOg) and triethylamine (3.8g) were added. The resulting reaction mixture further stirred for 2 hrs. After completion of the reaction, water was added and stirred for 10 min. aqueous layer was separated and washed with methylene chloride. The organic layer was acidified to pH 6-7 with 2N hydrochloric acid and finally the organic layer was concentrated to get desired product. The product was purified and dried to yield Rivaroxaban.
Yield: 10.0 gm
Purity: 99.3 %
EXAMPLE 8: One pot process for Rivaroxaban
Exemplified procedure in example 7 with the replacement of solvent ethyl acetate and base potassium hydroxide were used to get the rivaroxaban.
EXAMPLE 9: One pot process for Rivaroxaban
Exemplified procedure in example 7 with the replacement of solvent acetonitile and base potassium carbonate were used, methylene chloride was added in the reaction mixture to extract the Rivaroxaban.
…………..
WO 01/47919 discloses ー species from 4_ (4_ aminophenyl) -3_ morpholinone (I) Preparation of rivaroxaban approach:
…………..
US 07/149522 discloses ー kind to 5_ chlorothiophenes _2_ carbonyl chloride (IV) is a method for preparing raw rivaroxaban in:
………….
http://www.google.com/patents/CN102786516A?cl=en
Preparation 6 rivaroxaban implementation
The 12.5 g (76.9 mmol) 5- chloro-thiophene-2-carboxylic acid was suspended in 35 g of toluene was heated to 80 で, at this temperature, a solution of 11.0 g (92.5 mmol) of thionyl chloride, reaction was continued for 30 min; then warmed to the boiling point of toluene was 120 ° C, and stirring was continued under reflux until cessation of gas; cooled to room temperature, the reaction mixture was concentrated under reduced pressure to remove excess thionyl chloride and toluene to give 5-chloro-thiophene-2-carbonyl chloride;
The 11.6 g (37.0 mmol) 4- {4 – [(5S) -5- (aminomethyl) -2-oxo-1,3-oxazolidin-3-yl] phenyl} morpholin-3 -one hydrochloride was added 40ml of water, was added 4. 64 g (43 8 mmol.) Na2CO3 stirred and dissolved; then added 50 ml of toluene, was added dropwise at 10 ° C under the mixture, the mixture is 8. 0 g ( 44. 4 mmol) 5- chloro-thiophene-2-carbonyl chloride was dissolved in 15 ml of toluene, 20 min the addition was complete, then stirring was continued at room temperature, TLC monitoring progress of the reaction, 2 h after completion of the reaction; and the filter cake washed with water and washed with acetone to give a pale yellow solid 19. 6 g, used directly ko acid recrystallization, as a white solid 15. 2 g,
mp 227. 2 – 228. 1 ° C, [a] D21 = -38 2 ° (. c = 0. 30, DMS0), rivaroxaban yield of 94%, the total yield of 87.5% 0
1H-NMR (DMSO) 8: 3. 61 (. 2 H, t, / = 5 4 Hz), 3. 71 (2 H, t, / = 5 4 Hz.), 3.85 (IH, m ), 3.97 (2 H, t, J = 4. 5 Hz), 4. 19 (3 H, t, / = 7. 5 Hz), 4.84 (IH, m), 7. 19 (IH, d, / = 4. 2Hz), 7.40 (2 H, d, /=9.0 Hz), 7. 57 (2 H, t, /=9.0 Hz), 7. 69 (IH, d, J = 4. 19 Hz), 8. 96 (IH, t, / = 5. 7 Hz).
…………………
WO2013120465
EXAMPLE 28 (preparation of rivaroxaban)
10 g of the salt prepared according to Example 18 were suspended in 75 ml of N- methylpyrolidone, the suspension was heated at 50°C, then 14 ml of triethylamine was added and the mixture was heated at 60°C. This was followed by addition of 15.7 ml of a solution of 5-chlorothiophene-2-carboxylic acid chloride in toluene (2.46 M) and the reaction mixture was stirred and heated at 55°C for 15 minutes, then slowly cooled below 30°C, 75 ml were added and the turbid solution was filtered. The clear filtrate was stirred at 50°C, which was followed by addition of 15 ml of water and 75 ml of ethanol and stirring for 1 hour under slow cooling. The separated product was filtered off, washed with water (15 ml, 60°C), ethanol (2 x 25 ml) and dried in vacuo. 9.1 g (yield 81%) of rivaroxaban in the form of an off-white powder with the melt, point of 229.5-231°C was obtained, HPLC 99.95%, content of the ( )-isomer below 0.03%.
1H NMR (250 MHz, DMSO-D6), δ (ppm): 3.61 (t, 2H, CH2); 3.71 (m, 2H, CH2); 3.85 and 4.19 (m, 2×1 H, CH2); 3.97 (m, 2H, CH2); 4.19 (s, 2H, CH2); 4.84 (pent, 1H, CH); 7.18 (d, 1H); 7.40 (m, 2H); 7.56 (m, 2H); 7.68 (d, 1H); 8.95 (bt, 1H, NH).
13C NMR (250 MHz, DMSO-D6), δ (ppm): 42.2; 47.4; 49.0; 63.4; 67.7; 71.3; 1 18.3; 125.9; 128.1 ; 128.4; 133.2; 136.4; 137.0; 138.4; 154.0; 160.8; 165.9.
MS (m/z): 436.0729 (M+H)+. ation)
The optical isomer of rivaroxaban with the (R)- configuration was obtained by a process analogous to Example 28 starting from the salt prepared according to Example 19. The yield was 76%, HPLC 99.90%, content of the (5)-isomer below 0.03%. The NMR and MS spectra were in accordance with Example 28.
……………………..
……………………
5- chloro-thiophene-2-chloride by condensation, bromide, with 4- (4-amino-phenyl) -3-morpholinone cyclization reaction rivaroxaban, the following reaction scheme :( References : W02005068456, US20070149522, DE10300111)
5- chloro-thiophene-2-chloride by condensation, oxidation, and 4- (4-amino-phenyl) -3-morpholinone cyclization reaction racemic rivaroxaban, since the epoxidation step is not give any stereoselectivity, the final chiral separation need to get rivaroxaban, the reaction scheme is as follows :( References: W0-0147919)
…………
4- (4- amino-phenyl) -3-morpholinone by condensation, cyclization, and potassium phthalimide after reaction with methyl chloroformate to give (S) -2 – hydroxy -3- (I, 3- dioxo – isoindoline-2-yl) propyl-4- (3-oxo –morpholino) phenyl carbamate, by condensation, methylamine and Ethanol action under profit rivaroxaban, the following reaction scheme (Ref: US20110034465):
……….
4- (4- amino-phenyl) -3-morpholinone (R) and – epichlorohydrin, in the DMF solvent phthalimide potassium salt was reacted with ammonia solution and then prepared to succeed amino compound, and 5-chloro-thiophene-2-chloride in pyridine catalyzed system benefit rivaroxaban, the following reaction scheme (Ref: W02009023233):
………….
4- (4- amino-phenyl) -3-morpholinone after condensation with (R) – epichlorohydrin, then the 5-chloro-thiophene-2-amide lithium chloride and tert-butyl the reaction of an alcohol potassium enrichment rivaroxaban, the following reaction scheme (Ref: US7816355):
……………….
3-chloro-1,2-propanediol by cyclization, the reaction with phthalimide, then with 4- (4-aminophenyl) -3-morpholinone reaction, CDI and hydrazine to give 4- {4- [(5S) -5- (aminomethyl) -2-oxo-1,3-oxazolidin-3-yl] phenyl} morpholin-3-one under the influence, in pyridine and under the action of tetrahydrofuran and 5-chloro-thiophene-2-chloride benefit rivaroxaban, the following reaction scheme (Reference: Gutcait, A. et al Tetrahedron:.. Asymmetry 1996, 7 (6), 1641-1648 Roehrig, .. S. et al J. Med Chem 2005,48 (19), 5900-5908)..:
…………..

http://www.google.com/patents/CN102702186A?cl=zh
Compound rivaroxaban Synthesis Example 7 formula (X), [0071] Example
[0072] Method One:
[0074] The compound of formula (VIII) of (180mg, 0. 618mmol), Ni chloride (5mL) and tris ko amine (187mg,
I. 85mmol) added to the reaction flask, stirred at room temperature for 10 minutes, cooled to 0 ° C, a solution of 5-chloro-2-thiophene chloride (224mg, 1.24mm0l), stirred at room temperature overnight; after the completion of the reaction, spin dry, rinse with anhydrous alcohol ko, filtered, washed ko anhydrous alcohol three times to obtain a white solid product rivaroxaban (215mg, embodiments of the total yield of 7,8 80%).
[0075] 1H-Mffi (DMSC) JOOMHz, δ d m):…. 3 61 (t, 2H, J = 5 6Hz), 3. 71 (t, 2H, J = 5 2Hz), 3 89 ( m, 1H), 3. 97 (t, 2H, J = 4. 4Hz), 4. 20 (m, 3H), 4. 85 (m, 1H), 7. 18 (d, 1H, J = 4. 0Hz), 7. 40 (d, 2H, J = 8. 8Hz), 7. 56 (d, 2H, J = 8. 8Hz), 7. 73 (d, 1H, J = 4. 0Hz).
The method of writing is:
[0078] The compound 5_ gas – oh -I- thiophene carboxylic acid (500mg, 3. 08mmol), MsCl (702mg, 6. 1 Bmmol) and sodium bicarbonate (. 517mg, 6 16mmol) was suspended in THF (20ml) in , heated to 60 ° C with stirring 45min, a large white suspension washed out; the reaction mixture was cooled to room temperature, the compound of formula VIII was added portionwise (800mg, 2 75mmol.), stirred for 5 hours, after completion of the reaction distilled THF, was added after the residue was cooled to room temperature, water (IOOml), at room temperature embrace Cheung 30min, filtered, and the filter cake washed with cold water, dried and added to a ko-ol (5ml) was heated at reflux for I hour. After cooling, stirred for 5 hours at room temperature After filtration to give the product of formula (X) of the compound rivaroxaban (719mg, 60%)
References
- “Xarelto: Summary of Product Characteristics”. Bayer Schering Pharma AG. 2008. Retrieved 2009-02-11.
- Abdulsattar, Y; Bhambri, R; Nogid, A (May 2009). “Rivaroxaban (xarelto) for the prevention of thromboembolic disease: an inside look at the oral direct factor xa inhibitor.”.P & T : a peer-reviewed journal for formulary management 34 (5): 238–44.PMID 19561868.
- Roehrig S, Straub A, Pohlmann J et al. (September 2005). “Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor”. Journal of Medicinal Chemistry 48 (19): 5900–8. doi:10.1021/jm050101d.PMID 16161994.
- Perzborn, Elisabeth; Roehrig, Susanne; Straub, Alexander; Kubitza, Dagmar; Misselwitz, Frank (17 December 2010). “The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor”. Nature Reviews Drug Discovery 10 (1): 61–75. doi:10.1038/nrd3185.
- “FDA Approves XARELTO® (rivaroxaban tablets) to Help Prevent Deep Vein Thrombosis in Patients Undergoing Knee or Hip Replacement Surgery” (Press release).Janssen Pharmaceutica. 2011-07-01. Retrieved 2011-07-01.
- Gómez-Outes, A; Terleira-Fernández, AI; Calvo-Rojas, G; Suárez-Gea, ML; Vargas-Castrillón, E (2013). “Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups.”. Thrombosis 2013: 640723. doi:10.1155/2013/640723. PMC 3885278.PMID 24455237.
- Brown DG, Wilkerson EC, Love WE (March 2015). “A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons”.Journal of the American Academy of Dermatology 72 (3): 524–34.doi:10.1016/j.jaad.2014.10.027. PMID 25486915.
- “Bayer’s Xarelto Approved in Canada” (Press release). Bayer. 2008-09-16. Retrieved2010-01-31.
- “Bayer’s Novel Anticoagulant Xarelto now also Approved in the EU” (Press release).Bayer. 2008-02-10. Retrieved 2010-01-31.
- “Medication Guide–Xarelto” (PDF). http://www.fda.gov/. U.S. Food and Drug Administration. Retrieved 1 September 2014.
- “Xarelto Side Effects”. http://www.webmd.com/. WebMD. Retrieved 1 September2014.
- “Xarelto Side Effects Center”. http://www.rxlist.com/. RxList. Retrieved 1 September2014.
- Eriksson BI, Borris LC, Dahl OE et al. (November 2006). “A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement”. Circulation 114 (22): 2374–81.doi:10.1161/CIRCULATIONAHA.106.642074. PMID 17116766.
- Eriksson BI, Borris LC, Friedman RJ et al. (June 2008). “Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty”. The New England Journal of Medicine 358(26): 2765–75. doi:10.1056/NEJMoa0800374. PMID 18579811.
- Kakkar AK, Brenner B, Dahl OE et al. (July 2008). “Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial”. Lancet 372 (9632): 31–9.doi:10.1016/S0140-6736(08)60880-6. PMID 18582928.
- Lassen MR, Ageno W, Borris LC et al. (June 2008). “Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty”. The New England Journal of Medicine358 (26): 2776–86. doi:10.1056/NEJMoa076016. PMID 18579812.
- Turpie A, Bauer K, Davidson B et al. “Comparison of rivaroxaban – an oral, direct factor Xa inhibitor – and subcutaneous enoxaparin for thromboprophylaxis after total knee replacement (RECORD4: a phase 3 study) / European Federation of National Associations of Orthopaedics and Traumatology Annual Meeting; May 29 – June 1, 2008; Nice, France, Abstract F85″. Journal of Bone & Joint Surgery, British Volume 92–B (SUPP II): 329.
- Turpie AG, Lassen MR, Davidson BL et al. (May 2009). “Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial”.Lancet 373 (9676): 1673–80. doi:10.1016/S0140-6736(09)60734-0. PMID 19411100.
- ClinicalTrials.gov. “Randomized, Double-Blind Study Comparing Once Daily Oral Rivaroxaban With Adjusted-Dose Oral Warfarin for the Prevention of Stroke in Subjects With Non-Valvular Atrial Fibrillation”. Retrieved 2009-02-11.
- ClinicalTrials.gov. “MAGELLAN – Multicenter, Randomized, Parallel Group Efficacy Superiority Study in Hospitalized Medically Ill Patients Comparing Rivaroxaban with Enoxaparin”. Retrieved 2009-02-11.
- ClinicalTrials.gov. “Once-Daily Oral Direct Factor Xa Inhibitor Rivaroxaban in the Long-Term Prevention of Recurrent Symptomatic Venous Thromboembolism in Patients With Symptomatic Deep-Vein Thrombosis or Pulmonary Embolism. The Einstein-Extension Study”. Retrieved 2009-02-11.
- ClinicalTrials.gov. “Oral Direct Factor Xa Inhibitor Rivaroxaban In Patients With Acute Symptomatic Deep-Vein Thrombosis (DVT) Without Symptomatic Pulmonary Embolism: Einstein-DVT Evaluation”. Retrieved 2009-02-11.
- ClinicalTrials.gov. “Oral Direct Factor Xa Inhibitor Rivaroxaban In Patients With Acute Symptomatic Pulmonary Embolism (PE) With Or Without Symptomatic Deep-Vein Thrombosis: Einstein-PE Evaluation”. Retrieved 2009-02-11.
- ClinicalTrials.gov. “A Randomized, Double-Blind, Placebo-Controlled, Event-Driven Multicenter Study to Evaluate the Efficacy and Safety of Rivaroxaban in Subjects With a Recent Acute Coronary Syndrome”. Retrieved 2009-02-11.
- “Venous Thromboembolic Event (VTE) Prophylaxis in Medically Ill Patients (MAGELLAN)”. ClinicalTrials.gov. 11 March 2011. Retrieved 15 April 2011.
- Hughes, Sue (5 April 2011). “MAGELLAN: Rivaroxaban prevents VTE in medical patients, but bleeding an issue”. theheart.org. Retrieved 15 April 2011.
- “About the MAGELLAN Study”. Bayer HealthCare. Retrieved 15 April 2011.
- Bauersachs, M.D., Rupert; The EINSTEIN Investigators (December 23, 2010). “Oral Rivaroxaban for Symptomatic Venous Thromboembolism”. The New England Journal of Medecine 363 (26): 2499–2510. doi:10.1056/NEJMoa1007903. PMID 21128814. Retrieved 4 April 2011.
- “Oral Direct Factor Xa Inhibitor Rivaroxaban In Patients With Acute Symptomatic Deep-Vein Thrombosis Without Symptomatic Pulmonary Embolism: Einstein-DVT Evaluation”. clinicaltrials.gov. Retrieved 15 April 2011.
- European Medicines Agency (2008). “CHP Assessment Report for Xarelto (EMEA/543519/2008)” (PDF). Retrieved 2009-06-11.
- Turpie AG (January 2008). “New oral anticoagulants in atrial fibrillation”. European Heart Journal 29 (2): 155–65. doi:10.1093/eurheartj/ehm575. PMID 18096568.
| WO2013120465A1 * | Feb 18, 2013 | Aug 22, 2013 | Zentiva, K.S. | A process for the preparation of rivaroxaban based on the use of (s)-epichlorohydrin |
| WO2001047919A1 | Dec 11, 2000 | Jul 5, 2001 | Bayer Ag | Substituted oxazolidinones and their use in the field of blood coagulation |
| WO2004060887A1 | Dec 24, 2003 | Jul 22, 2004 | Bayer Healthcare Ag | Method for producing 5-chloro-n-({5s)-2-oxo-3-[4-(3-oxo-4-morpholinyl)-phenyl]-1,3-oxazolidin-5-yl}-methyl)-2-thiophene carboxamide |
| WO2007116284A1 | Mar 26, 2007 | Oct 18, 2007 | Pfizer Prod Inc | Process for preparing linezolid |
| WO2009023233A1 | Aug 14, 2008 | Feb 19, 2009 | Concert Pharmaceuticals Inc | Substituted oxazolidinone derivatives |
| WO2010043110A1 | Oct 9, 2009 | Apr 22, 2010 | Changzhou Multiple Dimension Institute Of Industry Technology Co., Ltd. | A preparation method of high-purity l-carnitine |
| WO2010082627A1 | Jan 15, 2010 | Jul 22, 2010 | Daiso Co., Ltd. | Process for producing 2-hydroxymethylmorpholine salt |
| WO2010124835A1 | Apr 27, 2010 | Nov 4, 2010 | Belte Ag | Aluminium-silicon diecasting alloy for thin-walled structural components |
| WO2011080341A1 | Jan 3, 2011 | Jul 7, 2011 | Enantia, S.L. | Process for the preparation of rivaroxaban and intermediates thereof |
| WO2011098501A1 | Feb 10, 2011 | Aug 18, 2011 | Sandoz Ag | Method for the preparation of rivaroxaban |
| WO2011102640A2 | Feb 16, 2011 | Aug 25, 2011 | Hanmi Holdings Co., Ltd. | Method for preparing sitagliptin and amine salt intermediates used therein |
| WO2012159992A1 * | May 18, 2012 | Nov 29, 2012 | Interquim, S.A. | Process for obtaining rivaroxaban and intermediate thereof |
| CN102786516A * | Aug 21, 2012 | Nov 21, 2012 | 湖南师范大学 | Method for synthesizing rivaroxaban |
| US7157456 | Dec 11, 2000 | Jan 2, 2007 | Bayer Healthcare Ag | Substituted oxazolidinones and their use in the field of blood coagulation |
| US7816355 * | Apr 28, 2009 | Oct 19, 2010 | Apotex Pharmachem Inc | Processes for the preparation of rivaroxaban and intermediates thereof |
| US20110034465 | Feb 10, 2011 | Apotex Pharmachem Inc. | Processes for the preparation of rivaroxaban and intermediates thereof |
| CN101560209A * | Apr 15, 2008 | Oct 21, 2009 | Shenyang, one hundred million Leo Pharmaceutical Co., Ltd. | Pyrimidine oxazolidinone compound and preparation method comprising |
| CN101619061A * | Aug 11, 2009 | Jan 6, 2010 | Shenyang Pharmaceutical University | Cyanopyridyl substituted oxazolidinone compounds |
| CN101821260A * | Aug 14, 2008 | Sep 1, 2010 | Consett Pharmaceuticals Ltd. | Substituted oxazolidinone derivatives |
| CN102250076A * | May 27, 2011 | Nov 23, 2011 | Hengdian Group homes Chemical Co., Ltd. | One kind of rivaroxaban Rivaroxaban intermediates and preparation methods |
| CN102250077A * | Jun 15, 2011 | Nov 23, 2011 | Zhejiang University | A method for intermediate and rivaroxaban Rivaroxaban for the synthesis of |
| CN102311400A * | Jun 29, 2010 | Jan 11, 2012 | Xiang really Biotechnology Co., Ltd. | Aminomethyl-3-aryl-2-oxazolidinone class method – Preparation of L-5- |
| CN102320988A * | Jun 3, 2011 | Jan 18, 2012 | Shanghai Institute of Organic Chemistry | 4- (4-aminophenyl) -3-morpholinone intermediate amide, the synthesis method and uses |
| EP2354128A1 * | Feb 10, 2010 | Aug 10, 2011 | Sandoz Ag | Method for the preparation of rivaroxaban |
| WO2010124385A1 * | Apr 28, 2010 | Nov 4, 2010 | Apotex Pharmachem Inc. | Processes for the preparation of rivaroxaban and intermediates thereof |
FROM THE NET
RIVAROXABAN 5-Chloro-N-{[(5S) 2-oxo-3 [4-(3-oxo-4 …
32 mins ago – RIVAROXABAN 5-Chloro-N-{[(5S) 2-oxo-3 [4-(3-oxo-4-morpholinophenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide (Rivaroxaban) (1):1 rivaroxaban 1 …
WO 2015104605.new patent on Rivaroxaban, Wockhardt …
1 hour ago – WO 2015104605.new patent on Rivaroxaban, Wockhardt Ltd Process for preparing rivaroxaban – comprising the reaction of a thioester compound and its salts …

 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
(S)-5-chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl)
phenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide |
|
| Clinical data | |
| Trade names | Xarelto |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Licence data | EMA:Link, US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
oral |
| Pharmacokinetic data | |
| Bioavailability | 80% to 100%; Cmax = 2 – 4 hours (10 mg oral)[1] |
| Metabolism | CYP3A4 , CYP2J2 and CYP-independent mechanisms[1] |
| Biological half-life | 5 – 9 hours in healthy subjects aged 20 to 45[1][2] |
| Excretion | 2/3 metabolized in liver and 1/3 eliminated unchanged[1] |
| Identifiers | |
| CAS Registry Number | 366789-02-8  |
| ATC code | B01AX06 |
| PubChem | CID: 6433119 |
| IUPHAR/BPS | 6388 |
| DrugBank | DB06228  |
| ChemSpider | 8051086  |
| UNII | 9NDF7JZ4M3  |
| ChEMBL | CHEMBL198362  |
| Synonyms | Xarelto, BAY 59-7939 |
| Chemical data | |
| Formula | C19H18ClN3O5S |
| Molecular mass | 435.882 g/mol |

Rivaroxaban, a FXa inhibitor, is the active ingredient in XARELTO Tablets with the chemical name 5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5yl}methyl)-2-thiophenecarboxamide. The molecular formula of rivaroxaban is C19H18ClN3O5S and the molecular weight is 435.89. The structural formula is:
 |
Rivaroxaban is a pure (S)-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. Rivaroxaban is only slightly soluble in organic solvents (e.g., acetone, polyethylene glycol 400) and is practically insoluble in water and aqueous media.
Each XARELTO tablet contains 10 mg, 15 mg, or 20 mg of rivaroxaban. The inactive ingredients of XARELTO are: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. Additionally, the proprietary film coating mixture used for XARELTO 10 mg tablets is Opadry® Pink and for XARELTO 15 mg tablets is Opadry® Red, both containing ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide, and for XARELTO 20 mg tablets is Opadry® II Dark Red, containing ferric oxide red, polyethylene glycol 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
////////
Filed under: Uncategorized Tagged: BAY 59-7939, Ривароксабан, RIVAROXABAN, SPECTRAL, XARELTO, 利伐沙班, ريفاروكسابان






















 amcrasto@gmail.com
amcrasto@gmail.com