
RS-10085-197
SPM-925
RS-10085 (free base)

Pharmacology
Moexipril is available as a prodrug moexipril hydrochloride, and is metabolized in the liver to form the pharmacologically active compound moexiprilat. Formation of moexiprilat is caused by hydrolysis of an ethyl ester group.[5] Moexipril is incompletely absorbed after oral administration, and its bioavailability is low.[6] The long pharmacokinetic half-life and persistent ACE inhibition of moexipril allows once-daily administration.[7]
Moexipril is highly lipophilic,[2] and is in the same hydrophobic range as quinapril, benazepril, and ramipril.[7] Lipophilic ACE inhibitors are able to penetrate membranes more readily, thus tissue ACE may be a target in addition to plasma ACE. A significant reduction in tissue ACE (lung, myocardium, aorta, and kidney) activity has been shown after moexipril use.[8]
It has additional PDE4-inhibiting effects.[9]
Side effects
Moexipril is generally well tolerated in elderly patients with hypertension.[10] Hypotension, dizziness, increased cough, diarrhea, flu syndrome, fatigue, and flushing have been found to affect less than 6% of patients who were prescribed moexipril.[3][10]
Mechanism of action
As an ACE inhibitor, moexipril causes a decrease in ACE. This blocks the conversion of angiotensin I to angiotensin II. Blockage of angiotensin II limits hypertension within the vasculature. Additionally, moexipril has been found to possess cardioprotective properties. Rats given moexipril one week prior to induction of myocardial infarction, displayed decreased infarct size.[11] The cardioprotective effects of ACE inhibitors are mediated through a combination of angiotensin II inhibition and bradykininproliferation.[8][12] Increased levels of bradykinin stimulate in the production of prostaglandin E2[13] and nitric oxide,[12] which cause vasodilation and continue to exert antiproliferative effects.[8] Inhibition of angiotensin II by moexipril decreases remodeling effects on the cardiovascular system. Indirectly, angiotensin II stimulates of the production of endothelin 1 and 3 (ET1, ET3)[14] and the transforming growth factor beta-1 (TGF-β1),[15] all of which have tissue proliferative effects that are blocked by the actions of moexipril. The antiproliferative effects of moexipril have also been demonstrated by in vitro studies where moexipril inhibits the estrogen-stimulated growth of neonatal cardiac fibroblasts in rats.[12] Other ACE inhibitors have also been found to produce these actions, as well.
WO 2014202659
http://www.google.com/patents/WO2014202659A1?cl=en
US4344949
http://www.google.co.in/patents/US4344949
References
- Hochadel, Maryanne, ed. (2006). The AARP Guide to Pills. Sterling Publishing Company. p. 640. ISBN 978-1-4027-1740-6. Retrieved2009-10-09.
- Belal, F.F, K.M. Metwaly, and S.M. Amer. “Development of Membrane Electrodes for the Specific Determination of Moexipril Hydrochloride in Dosage Forms and Biological Fluids.” Portugaliae Electrochimica Acta. 27.4 (2009): 463-475.
- Rodgers, Katie, Michael C Vinson, and Marvin W Davis. “Breakthroughs: New drug approvals of 1995 — part 1.” Advanstar Communications, Inc. 140.3 (1996): 84.
- Dart, Richard C. (2004). Medical toxicology. Lippincott Williams & Wilkins. p. 647. ISBN 978-0-7817-2845-4. Retrieved 2009-10-09.
- Kalasz, H, G. Petroianu, K. Tekes, I. Klebovich, K. Ludanyi, et al. “Metabolism of moexipril to moexiprilat: determination of in vitro metabolism using HPLC-ES-MS.” Medicinal Chemistry. 3 (2007): 101-106.
- Jump up^ Chrysant, George S, PK Nguyen. “Moexipril and left ventricular hypertrophy.” Vascular Health Risk Management. 3.1 (2007): 23-30.
- Cawello W, H. Boekens, J. Waitzinger, et al. “Moexipril shows a long duration of action related to an extended pharmacokinetic half-life and prolonged ACE-inhibition.” Int J Clin Pharmacol Ther. 40 (2002): 9-17.
- ^ Jump up to:a b c Chrysant, SG. “Vascular remodeling: the role of angiotensin-converting enzyme inhibitors.” American Heart Journal. 135.2 (1998): 21-30.
- Jump up^ Cameron, RT; Coleman, RG; Day, JP; Yalla, KC; Houslay, MD; Adams, DR; Shoichet, BK; Baillie, GS (May 2013). “Chemical informatics uncovers a new role for moexipril as a novel inhibitor of cAMP phosphodiesterase-4 (PDE4)”. Biochemical Pharmacology 85 (9): 1297–1305. doi:10.1016/j.bcp.2013.02.026. PMC 3625111. PMID 23473803.
- White, WB, and M Stimpel. “Long-term safety and efficacy of moexipril alone and in combination with hydrochlorothiazide in elderly patients with hypertension.” Journal of human hypertension. 9.11 (1995): 879-884.
- Rosendorff, C. “The Renin-Angiotensin System and Vascular Hypertrophy.” Journal of the American College of Cardiology. 28 (1996): 803-812.
- Hartman, J.C. “The role of bradykinin and nitric oxide in the cardioprotective action of ACE inhibitors.” The Annals of Thoracic Surgery. 60.3 (1995): 789-792.
- Jaiswal, N, DI Diz, MC Chappell, MC Khosia, CM Ferrario. “Stimulation of endothelial cell prostaglandin production by angiotensin peptides. Characterization of receptors.” Hypertension. 19.2 (1992): 49-55.
- Phillips, PA. “Interaction between endothelin and angiotensin II.” Clinical and Experimental Pharmacology and Physiology. 26.7. (1999): 517-518.
- Youn, TJ, HS Kim, BH Oh. “Ventricular remodeling and transforming growth factor-beta 1 mRNA expression after nontransmural myocardial infarction in rats: effects of angiotensin converting enzyme inhibition and angiotensin II type 1 receptor blockade.” Basic research in cardiology. 94.4 (1999): 246-253.
////////////
| Systematic (IUPAC) name | |
|---|---|
|
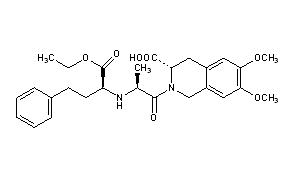
(3S)-2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
|
|
| Clinical data | |
| Trade names | Univasc |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a695018 |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Bioavailability | 13-22% |
| Protein binding | 90% |
| Metabolism | Hepatic (active metabolite, moexiprilat) |
| Biological half-life | 1 hour; 2-9 hours (active metabolite) |
| Excretion | 50% (faeces), 13% (urine) |
| Identifiers | |
| CAS Registry Number | 103775-10-6  |
| ATC code | C09AA13 |
| PubChem | CID: 91270 |
| IUPHAR/BPS | 6571 |
| DrugBank | DB00691  |
| ChemSpider | 82418  |
| UNII | WT87C52TJZ  |
| KEGG | D08225  |
| ChEMBL | CHEMBL1165  |
| Chemical data | |
| Formula | C27H34N2O7 |
| Molecular mass | 498.568 g/mol |
Filed under: Uncategorized Tagged: moexipril