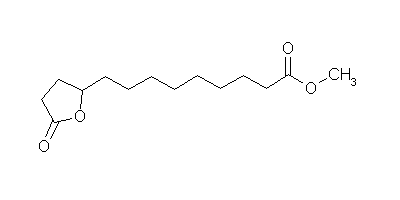
9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester
|
![353]() |
|
|
|
| Name |
9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester |
| Synonyms |
|
| Name in Chemical Abstracts |
2-Furannonanoic acid, tetrahydro-5-oxo-, methyl ester |
| CAS No |
22623-86-5 |
|
|
| Molecular formula |
C14H24O4 |
| Molecular mass |
256.35 |
| SMILES code |
O=C1OC(CC1)CCCCCCCCC(=O)OC |
1H NMR
![1H NMR]()
| 1H-NMR: 9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester |
| 500 MHz, CDCl3 |
| delta [ppm] |
mult. |
atoms |
assignment |
| 1.24-1.45 |
m |
10 H |
4-H, 5-H, 6-H, 7-H, 8-H |
| 1.57 |
m |
2 H |
3-H |
| 1.70 |
m |
1 H |
9-H |
| 1.82 |
m |
1 H |
9-H |
| 2.27 |
t |
2 H |
2-H |
| 2.30 |
m |
2 H |
3-H (ring) |
| 2.50 |
m |
2 H |
4-H (ring) |
| 3.67 |
s |
3 H |
O-CH3 |
| 4.48 |
m |
1 H |
2-H (ring) |
|
|
|
|
![NMR XXX]()
13C NMR
![13C NMR]()
| 13C-NMR: 9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester |
| 125.7 MHz, CDCl3 |
| delta [ppm] |
assignment |
| 24.9 |
C3 |
| 25.2 |
C9 |
| 28.0-29.2 |
C4, C5, C6, C7, C8, C3 (ring) |
| 34.0 |
C2 |
| 35.5 |
C4 (ring) |
| 51.4 |
O-CH3 |
| 81.0 |
C2 (ring) |
| 174.2 |
C1 (O-C(=O)-) |
| 177.2 |
C5 (O-C(=O)-, ring) |
| 76.5-77.5 |
CDCl3 |
![13C XXX]()
![IR]()
| IR: 9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester |
| [Film, T%, cm-1] |
| [cm-1] |
assignment |
| 2931, 2856 |
aliph. C-H valence |
| 1776 |
C=O valence, lactone |
| 1737 |
C=O valence, ester |
|
|
Synthesis of 9-(5-oxotetrahydrofuran-2-yl)nonanoic acid methyl ester
|
| Reaction type: |
addition to alkenes, radical reaction, ring closure reaction |
| Substance classes: |
alkene, halogencarboxylic acid ester, lactone |
| Techniques: |
working with cover gas, stirring with magnetic stir bar, heating under reflux, evaporating with rotary evaporator, filtering, recrystallizing, heating with oil bath |
| Degree of difficulty: |
Easy |
Operating scheme
![Operating scheme]() Instructions
Instructions
http://www.oc-praktikum.de/nop/en/instructions/pdf/4005_en.pdf
Instruction (batch scale 100 mmol)
Equipment 250 mL two-neck flask, protective gas supply, reflux condenser, heatable magnetic stirrer, magnetic stir bar, rotary evaporator, Buechner funnel, suction flask, desiccator, oil bath Substances undecenoic acid methyl ester (bp 248 °C) 19.8 g (22.3 mL, 100 mmol) iodoacetic acid ethyl ester (bp 73-74 °C/ 21 hPa) 27.8 g (15.4 mL, 130 mmol) copper powder (finely powdered, >230 mesh ASTM) 30.5 g (480 mmol) tert-butyl methyl ether (bp 55 °C) 130 mL petroleum ether (bp 60-80 °C) 300 mL Reaction In a 250 mL two-neck flask with magnetic stir bar and a reflux condenser connected with a protective gas piping 19.8 g (22.3 mL, 100 mmol) undecenoic acid methyl ester and 27.8 g (15.4 mL, 130 mmol) iodoacetic acid ethyl ester are mixed with 30.5 g (480 mmol) copper powder under a protective gas atmosphere. Afterwards the reaction mixture is stirred at 130 °C oil bath temperature under protective gas for 4 hours. (Reaction monitoring see Analytics.)
Work up The reaction mixture is cooled down to room temperature, 30 mL tert-butyl methyl ether are added, the mixture is stirred for 5 minutes and filtered off. The copper powder on the filter is washed four times with 25 mL tert-butyl methyl ether each. Filtrates and wash solutions are combined, the solvent is evaporated at the rotary evaporator. A yellow oil remains as crude product. Crude yield: 25.4 g.
The crude product is dissolved in 300 mL petroleum ether under reflux. The solution is allowed to cool down to room temperature, then it is stored in the refrigerator over night for complete crystallization. The crystalline product is sucked off over a Buechner funnel and dried in the vacuum desiccator. The mother liquor is stored again in the refrigerator for a check of complete crystallization. Yield: 19.5 g (76.1 mmol, 76%); white solid, mp 34 °C Comments In order to achieve a quantitative reaction within 4 hours, a fivefold excess of copper is used.
Waste management Recycling The copper powder can be used three times.
Waste disposal Waste Disposal evaporated tert-butyl methyl ether (might contain iodoethane) organic solvents, containing halogen mother liquor from recrystallization organic solvents, containing halogen copper powder solid waste, free from mercury, containing heavy metals
Time 6-7 hours
Break After heating and before recrystallizing
Degree of difficulty Easy
Analytics Reaction monitoring with TLC Sample preparation: Using a Pasteur pipette, two drops of the reaction mixture are taken and diluted with 0.5 mL diethyl ether. TLC-conditions: adsorbant: TLC-aluminium foil (silica gel 60) eluent: petroleum ether (60/80) : acetic acid ethyl ester = 7 : 3 visualisation: The TLC-aluminium foil is dipped in 2 N H2SO4 and then dried with a hot air dryer. Reaction monitoring with GC Sample preparation: Using a Pasteur pipette, one drop of the reaction mixture is taken and diluted with 10 mL dichloromethane. From this solution, 0.2 µL are injected. 10 mg from the solid product are dissolved in 10 mL dichloromethane. From this solution, 0.2 µL are injected. GC-conditions: column: DB-1, 28 m, internal diameter 0.32 mm, film 0.25 µm inlet: on-column-injection carrier gas: hydrogen (40 cm/s) oven: 90 °C (5 min), 10 °C/min to 240 °C (40 min) detector: FID, 270 °C Percent concentration was calculated from peak areas.
![crude product chromatogram]()
| GC: crude product |
| column |
DB-1, L=28 m, d=0.32 mm, film=0.25 µm |
| inlet |
on column injection, 0.2 µL |
| carrier gas |
H2, 40 cm/s |
| oven |
90°C (5 min), 10°C/min –> 240°C (40 min) |
| detector |
FID, 270°C |
| integration |
percent concentration calculated from relative peak area |
![pure product chromatogram]()
| GC: pure product |
| column |
DB-1, L=28 m, d=0.32 mm, film=0.25 µm |
| inlet |
on column injection, 0.2 µL |
| carrier gas |
H2, 40 cm/s |
| oven |
90°C (5 min), 10°C/min –> 240°C (40 min) |
| detector |
FID, 270°C |
| integration |
percent concentration calculated from relative peak area |
| Batch scale: |
|
0.01 mol |
|
0.1 mol |
10-Undecenoic acid methyl ester |
| Educts |
|
Amount |
Risk |
Safety |
| 10-Undecenoic acid methyl ester |
|
19.8 g |
H- EUH- |
P- |
| Iodoacetic acid ethyl ester |
![Toxic GHS06]() |
![Corrosive GHS05]() |
Danger |
|
27.8 g |
H300 H314 EUH- |
P264 P280 P305 + 351 + 338 P310 |
| Reagents |
|
Amount |
Risk |
Safety |
| Copper powder |
![Dangerous for the environment GHS09]() |
Warning |
|
30.5 g |
H400 EUH- |
P273 |
| Solvents |
|
Amount |
Risk |
Safety |
| tert-Butyl methyl ether |
![Flammable GHS02]() |
![Harmful GHS07]() |
Danger |
|
130 mL |
H225 H315 |
P210 |
| Petroleum ether (60-80) |
|
300 mL |
H225 H304 H315 H336 H411 EUH- |
P210 P261 P273 P301 + 310 P331 |
| Others |
|
Amount |
Risk |
Safety |
| Sulfuric acid 2N |
![Corrosive GHS05]() |
Danger |
|
H314 H290 EUH- |
P280 P301 + 330 + 331 P305 + 351 + 338 P309 + 310 |
| Solvents for analysis |
|
Amount |
Risk |
Safety |
| Petroleum ether (60-80) |
|
H225 H304 H315 H336 H411 EUH- |
P210 P261 P273 P301 + 310 P331 |
| Acetic acid ethyl ester |
![Flammable GHS02]() |
![Harmful GHS07]() |
Danger |
|
H225 H319 H336 EUH066 |
P210 P261 P305 + 351 + 338 |
| Dichloromethane |
![Dangerous to the organs, mutagenic, cancerogenic, toxic to reproduction GHS08]() |
![Harmful GHS07]() |
Warning |
|
H351 H315 H319 H335 H336 H373 |
P261 P281 P305 + 351 + 338 |
| Batch scale: |
|
0.01 mol |
|
0.1 mol |
10-Undecenoic acid methyl ester |
| Products |
|
Amount |
Risk |
Safety |
| 9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester |
| Batch scale: |
|
0.01 mol |
|
0.1 mol |
10-Undecenoic acid methyl ester |
![two-necked flask 250 mL two-necked flask 250 mL]() |
two-necked flask 250 mL |
|
![protective gas piping protective gas piping]() |
protective gas piping |
![reflux condenser reflux condenser]() |
reflux condenser |
|
![heatable magnetic stirrer with magnetic stir bar heatable magnetic stirrer with magnetic stir bar]() |
heatable magnetic stirrer with magnetic stir bar |
![rotary evaporator rotary evaporator]() |
rotary evaporator |
|
![suction filter suction filter]() |
suction filter |
![suction flask suction flask]() |
suction flask |
|
![exsiccator with drying agent exsiccator with drying agent]() |
exsiccator with drying agent |
![oil bath oil bath]() |
oil bath |
|
Simple evaluation indices
|
| Batch scale: |
|
0.01 mol |
|
0.1 mol |
10-Undecenoic acid methyl ester |
| Atom economy |
|
53.9 |
% |
| Yield |
|
76 |
% |
| Target product mass |
|
19.5 |
g |
| Sum of input masses |
|
370 |
g |
| Mass efficiency |
|
53 |
mg/g |
| Mass index |
|
19 |
g input / g product |
| E factor |
|
18 |
g waste / g product |
………………
………
Filed under:
spectroscopy,
SPOTLIGHT,
SYNTHESIS Tagged:
9-(5-Oxotetrahydrofuran-2-yl)nonanoic acid methyl ester ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()







 Instructions
Instructions