Image may be NSFW.
Clik here to view.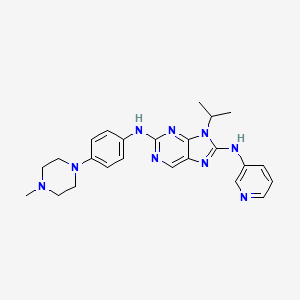
SKLB 1028
IND Filed
A multi-targeted inhibitor potentially for the treatment of leukemia and non small cell lung cancer.
Image may be NSFW.
Clik here to view.
SKLB-1028
CAS 1350544-93-2
9-isopropyl-N2-(4-(4-methylpiperazin-1-yl)phenyl)-N8-(pyridin-3-yl)-9H-purine- 2,8-diamine
2-N-[4-(4-methylpiperazin-1-yl)phenyl]-9-propan-2-yl-8-N-pyridin-3-ylpurine-2,8-diamine
9-Isopropyl-N2-[4-(4-methylpiperazin-1-yl)phenyl]-N8-(3-pyridyl)-9H-purine-2,8-diamine, 443.5474, C24H29N9, Preclinical
9-isopropyl-N2-(4-(4-methylpiperazin-1-yl)phenyl)-N8-(pyridin-3-yl)-9H-purine- 2,8-diamine. Yield 65.6 %. HPLC>98.6%. 1H NMR(400 MHz, DMSO-d6): δ 9.22(s, 1H), 9.05(s, 1H), 8.94(d, J=2.8Hz, 1H), 8.39(s, 1H), 8.34(d, J=8.4Hz, 1H), 8.20(m, 1H), 7.63(d, J=8.8Hz, 2H), 7.37(m, 1H), 6.88 (d, J=8.8Hz, 2H), 4.88(m, 1H), 3.05(m, 4H), 2.45(m, 4H), 2.22(s, 3H), 1.69(s, 3H), 1.68(s, 3H)ppm。HRMS (ESI) m/z [M-H]- calcd for C24H29N9: 443.2546, found: 442.2538………..Leukemia (2012), 26(8)
PATENT
Synthetic route is as follows:
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Example reaction is as follows:
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
8
Image may be NSFW.
Clik here to view.
Preparation of chloro-4-amino-5-nitro pyrimidine of Example 12-
Image may be NSFW.
Clik here to view.
Was added dropwise 2,4-dichloro-5-nitro-pyrimidine (lO Aqueous ammonia (8.0ml) and Ν, Ν- diisopropylethylamine (13.2ml) was dissolved in 150ml dichloromethane, 0 ° C when .Og) in dichloromethane (30ml) solution, after dropwise, maintaining the temperature of the reaction one hour, the precipitate was filtered off, the filter cake was recrystallized to give a yellow solid 8.1g, yield 90.1%
Product 1HNMR (400MHz, DMSO-i¾): δ 9.20 (s, 1H), 9.02 (s, 1H), 8.60 (s, lH) ppm
Preparation of pyrimidine
Image may be NSFW.
Clik here to view.
Isopropylamine (4.5ml) and Ν, Ν- diisopropylethylamine (13.2ml) was dissolved in 150ml of dichloromethane, was added dropwise 2,4-dichloro-5-nitro-pyrimidine at 0 ° C ( lO.Og) in dichloromethane (30ml) solution, after dropwise, maintaining the reaction temperature for half an hour, and purified by column chromatography to give a light yellow solid was 10.1g, 90.4% yield of product 1H NMR (400 MHz, CDCl 3 ): [delta] 9.03 (s, 1H), 8.24 (s, 1H), 4.53 (m, 1H), 1.34 (d, J = 6.8 Hz, 6H) ppm 0
Example 16, 4-amino-2- (4- (4-methyl-piperazin-1-yl) anilino) -5-nitro-pyrimidin embodiment
Image may be NSFW.
Clik here to view.
4- (4-methylpiperazine) aniline (3.8g) was added to the compound 2-l (3.5g) in n-butanol (150ml) solution, the reaction for 4.5 hours at 90 ° C, cooled to room temperature, filtered , washed, and dried to give a red solid (5.2g), a yield of 79.5%. Product ‘H NMR (400 MHz, CDCl 3 ): [delta] 9.07 (s, 1H), 8.52 (s, 2H), 8.40 (s, 1H), 7.57 (s, 1H), 7.51 (s, 1H), 7.10 (m, 2H), 3.3 l (t, J = 4.8Hz, 4H), 2.81 (t, J = 4.8Hz, 4H), 2.30 (s, 3H) ppm.
Example 90,
9-isopropyl-2- (4- (4-methyl-piperazin-1-yl) anilino) -8- (pyridin-3-yl) -9H- purine
The compound 5- 7 (2.05g) was dissolved in dichloromethane (90ml), were added sequentially EDCI (2.3g), Ν, Ν- diisopropylethylamine (4.9ml), 3- pyridyl isothiocyanate ester (1.0g), stirred at room temperature for half an hour, then refluxed for 10 hours, TLC monitoring completion of the reaction the raw material 5-7 was cooled and purified by column chromatography to give a light red solid, yield 65.7%.
Image may be NSFW.
Clik here to view.
Product ESI-MS (m / z,%) 442.26 (MH) -. Ή NMR (400 MHz, DMSO-d 6 ): [delta] 9.38 (s, IH), 9.13 (s, IH), 8.99 (s, IH), 8.40 (s, IH), 8.36 (d, J = 8.4 Hz, IH), 8.20 (d, J = 4.4Hz, IH), 7.70 (d, J = 8.8Hz, 2H), 7.37 (m, IH), 6.96 (d, J = 8.8Hz, 2H), 4.97-4.92 ( m, IH), 3.35 (s, 6H), 2.80 (s, 3H): 2.53 (s, 2H), 1.69 (s, 6H) ppm.
/////////SKLB 1028, IND Filed, Preclinical
CN1CCN(CC1)c5ccc(Nc3nc4n(C(C)C)c(Nc2cccnc2)nc4cn3)cc5
Filed under: Preclinical drugs, Uncategorized Tagged: preclinical Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.