Image may be NSFW.
Clik here to view.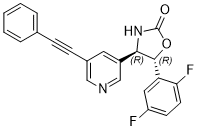
(4R,5R)-5-(2,5-difluorophenyl)-4-(5-(phenylethynyl)pyridin-3-yl)oxazolidin-2-one
cas 1375751-08-8
Chemical Formula: C22H14F2N2O2
Exact Mass: 376.1023
Bristol-Myers Squibb Company INNOVATOR
BMS-955829 is a Positive allosteric modulators (PAMs). BMS-955829 shows high functional PAM potency, excellent mGluR5 binding affinity, low glutamate fold shift, and high selectivity for the mGluR5 subtype. BMS-955829 is a potent mGluR5 PAM (EC50 = 2.6 ± 1.0 nM; n = 6), devoid of inherent mGluR5 agonist activity (EC50 > 30μM). The measured binding Ki of BMS-955829 was found to be 1.6 nM, which was in good agreement with its functional potency.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
SYNTHESIS AND INTERMEDIATES…….https://www.google.co.in/patents/WO2012064603A1?cl=en
Image may be NSFW.
Clik here to view.
Intermediate 73
Diethyl 2,5-difluorobenzylphosphonate. A mixture of 2-(bromomethyl)-l,4- difluorobenzene (3 g, 14.49 mmol) and triethyl phosphite (7.72 ml, 43.5 mmol) was heated to 160 °C with stirring for 4 hours, cooled to ambient temperature and concentrated under high vacuum to remove most triethyl phosphite. The resulting residue was purified by column chromatography (20% to 30 % EtO Ac/Toluene) providing diethyl 2,5-difluorobenzylphosphonate (3.76 g, 13.52 mmol, 93 % yield) as colorless oil. ¾ NMR (500MHz, DMSO-d6) δ 7.30 – 7.10 (m, 3H), 4.05 – 3.91 (m, 4H), 3.31 – 3.20 (m, 2H), 1.18 (t, J=7.0 Hz, 6H). MS Anal. Calcd. for [M+H]+ CiiHieFzOsP: 265.2; found 265.3.
Image may be NSFW.
Clik here to view.
Intermediate 74
(E)-3-Bromo-5-(2,5-difluorostyryl)pyridine. To a stirred solution of diethyl 2,5-difluorobenzylphosphonate (63.5 g, 240 mmol) and 5-bromonicotinaldehyde (50.7 g, 264 mmol) in tetrahydrofuran (1923 ml) was added potassium tert-butoxide in tetrahydrofuran (312 ml, 312 mmol) at -10 °C. After three hours, the reaction mixture was allowed to warm to ambient temperature and stirring was continued for another 16 hours at which time the reaction mixture was diluted with ether (800 mL) and washed with H2O. The organic layer was dried over anhydrous magnesium sulfate, filered and concentrated to provide a yellow wax to which was added 300 mL of hexane and after sonication filtered to provide (is)-3-bromo-5-(2,5- difluorostyryl)pyridine (54 g, 173 mmol, 72.1%) as a white solid. XH NMR
(500MHz, DMSO-d6) δ 8.78 (d, J=1.8 Hz, IH), 8.63 (d, J=2.1 Hz, IH), 8.44 (t, J=2.0 Hz, IH), 7.67 (ddd, J=9.4, 6.0, 3.2 Hz, IH), 7.56 – 7.48 (m, IH), 7.46 – 7.40 (m, IH), 7.34 (td, J=9.6, 4.6 Hz, IH), 7.24 (tt, J=8.3, 3.6 Hz, IH). MS Anal. Calcd. for [M+H]+ Ci3H9BrF2N: 296.0; found 298.1
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Intermediate 75
Tert-butyl (lR,2R)-l-(5-bromopyridin-3-yl)-2-(2,5-difluorophenyl)-2- hydroxyethylcarbamate. A solution of tert-butyl carbamate (4.18 g, 35.0 mmol) in propanol (39 ml) was sequentially treated with sodium hydroxide (1.376 g, 34.4 mmol) in water (72 ml) and tert-butyl hypochlorite (3.88 ml, 34.4 mmol). After 5 min of stirring, the reaction mixture was cooled to 0 °C. A solution of
(DHQD)2PHAL (0.555 g, 0.677 mmol) in propanol (39 ml), a solution of (E)-3- bromo-5-(2,5-difluorostyryl)pyridine (3.34 g, 11.28 mmol) in propanol (68 ml) , and potassium osmate dihydrate (0.166 g, 0.451 mmol) were sequentially added. The reaction mixture was stirred for three additional hours at 0 °C, warmed to ambient temperature and after an additional 16 hours the light yellow homogenous solution was quenched with saturated aqueous sodium sulfite (100 mL). The aqueous phase was extracted with ethyl acetate( 2 X 50 mL), the combined organic phases were washed with brine (100 mL), dried over anhydrous magnesium sulfate and concentrated to afford a residue which was purified via column chromatography (25% to 40 % EtO Ac/Hex) to provide tert-butyl (7R,2R)-l-(5-bromopyridin-3-yl)-2- (2,5-difluorophenyl)-2-hydroxyethylcarbamate (2.2991 g, 5.09 mmol, 45.1 % yield) as an optically enriched mixture of enantiomers. XH NMR (500MHz, DIVISOR) δ 8.56 (d, J=1.8 Hz, IH), 8.40 (s, IH), 8.03 (s, IH), 7.52 (d, J=9.5 Hz, IH), 7.25 (br. s., IH), 7.10 (t, J=5.6 Hz, 2H), 5.89 (d, J=4.9 Hz, IH), 5.03 (t, J=5.0 Hz, IH), 4.83 (dd, J=8.9, 5.2 Hz, IH), 1.40 – 1.34 (m, 9H), MS Anal. Calcd. for [M+H]+
Ci8H2oBrF2 203: 429.1; found 431.3.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Intermediate 77
(lR,2R)-2-Amino-2-(5-bromopyridin-3-yl)-l-(2,5-difluorophenyl)ethanol To a stirred solution of tert-butyl tert-butyl (7R,2R,)-l-(5-bromopyridin-3-yl)-2-(2,5- difluorophenyl)-2-hydroxyethylcarbamate (2.30 g, 5.09 mmol) in methylene chloride (30 mL) was added HC1 in dioxane (30 ml, 120 mmol). The reaction mixture was placed in an oil bath set to 50 °C. After three hours, the reaction mixture was concentrated providing (7R,2R^-2-amino-2-(5-bromopyridin-3-yl)-l-(2,5- difluorophenyl)ethanol 2HC1 salt (2.10 g, 4.97 mmol, 98 % yield) as an optically enriched yellow wax. XH NMR (500MHz, DMSO-d6) δ 8.95 (d, J=3.7 Hz, 2H), 8.64 (d, J=2.4 Hz, 1H), 8.45 (d, J=1.5 Hz, 1H), 8.31 (t, J=2.0 Hz, 1H), 7.47 – 7.09 (m, 3H), 7.04 (td, J=9.2, 4.4 Hz, 1H), 5.29 (d, J=9.2 Hz, 1H), 4.57 (dd, J=9.0, 5.3 Hz, 1H). Anal. Calcd. for [M+H]+ Ci3H12BrF2N20: 329.0; found 331.2.
Image may be NSFW.
Clik here to view.
Intermediate 78
(4R,5R)-4-(5-Bromopyridin-3-yl)-5-(2,5-difluorophenyl)oxazotidin-2-one. To optically enriched (7R,2R)-2-amino-2-(5-bromopyridin-3-yl)-l-(2,5- difluorophenyl)ethanol, 2 HC1 (2.019 g, 4.82 mmol) in tetrahydrofuran (98 ml) was added diisopropylethylamine (2.95 ml, 16.87 mmol) and the resultant solution was stirred for ten mintues at ambient temperature, cooled to 0 °C and
carbonyldiimidazole (1.094 g, 6.75 mmol) was added. After an additional three hours at 0 °C the reaction mixture was warmed to ambient temperature and allowed to stir for another 16 hours. 2M ¾ in methanol (5ml) was added and after ten mintues the suspension was filtered and concentrated to a pink oil which was purified by column chromatography (25% to 40 % EtO Ac/Hex) providing (4R,5R)-4-(5- bromopyridin-3-yl)-5-(2,5-difluorophenyl)oxazolidin-2-one (1.353 g, 3.62 mmol, 75 % yield) as an optically enriched white solid. ¾ NMR (500MHz, DMSO-d6) δ 8.80 – 8.68 (m, 1H), 8.55 (d, J=2.1 Hz, 2H), 8.16 (t, J=2.1 Hz, 1H), 7.46 – 7.28 (m, 3H), 5.71 – 5.58 (m, 1H), 5.02 (d, J=6.7 Hz, 1H). MS Anal. Calcd. for [M+H]+ Ci4H10BrF2 2O2: 355.0; found 357.2.
Intermediate 79
(4R,5R)-4-(5-Bromopyridin-3-yl)-5-(2,5-difluorophenyl)oxazotidin-2-one. Method – 2 A mixture of tert-butyl ((lR,2R)-l-(54oromopyridin-3-yl)-2-(2,5- difluorophenyl)-2-hydroxyethyl)carbamate and tert-butyl ((lR,2R)-2-(5- bromopyridin-3-yl)-l-(2,5-difluorophenyl)-2-hydroxyethyl)carbamate (about 6: 1 ratio) (101 g, 236 mmol) in tetrahydrofuran (590 mL) was cooled to -7 °C with a methanol/ice bath. To this mixture was added a solution of 1 M potassium tert- butoxide in tetrahydrofuran (590 mL, 590 mmol) via an addition funnel while maintaining the internal temperature < 3 °C. The reaction mixture was stirred with a cooling bath for 30 min and then allowed to warm up to room temperature. After 20 h, the reaction was deemed complete by LC/MS. The reaction mixture was concentrated to dryness to give crude product. Another identical scale reaction was performed. The crude products of the two batches were combined to work up together. They were treated with ethyl acetate (1.75 L) and water (1.75 L). The layers were separated. The organic layer was washed with brine (1.75 L), dried (sodium sulfate), and evaporated to give 161.5 g of crude product as a brown solid. This was purified by ISCO to give 67.1 g (42% yield). LC/MS (ES+) 355/357 (M+H, 100; Br isotope pattern); XH NMR (400MHz, CDCl3) δ 8.75 (d, J=2.2 Hz, 1H), 8.53 (d, J=1.8 Hz, 1H), 7.97 (t, J=2.0 Hz, 1H), 7.29 – 7.23 (m, 1H), 7.18 – 7.09 (m, 2H), 6.40 (s, 1H), 5.56 (d, J=5.7 Hz, 1H), 4.84 (d, J=5.5 Hz, 1H); Calcd for
Ci4H9N2BrF202: C, 47.34; H, 2.55; N, 7.86; Br, 22.50; F, 10.69. Found: C, 47.29; H, 2.61; N, 7.87; Br, 22.40; F, 10.37. Note: Chiral HPLC of the above sample showed 4.7% of the enantiomer. The (4S, 55) enantiomer can be purged by recrystallization from methanol to give > 99.9 ee with 67% recovery.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Scheme 1.
Pd(0)/Cu(l)/ TBAF Scheme 2.
cheme 4.
R’ = H, alkyl
Scheme 8.
cheme 11.
Scheme 12.
Scheme 14.
Scheme 15.
R” = H, alkyl R” = alkyl
cheme 16.
R’ = alky I
R” = alkyl
Scheme 17.
R’ = H, alkyl
R” = H, alkyl
Scheme 18.
R’ = H, alkyl R’ = H, alkyl
P T/US2011/059339
COMPD IS 185
Image may be NSFW.
Clik here to view.
Example 185
(4R, 5R)-5-(2, 5-difluorophenyl)-4-(5-(phenylethynyl)-3-pyridinyl)-l, 3-oxazolidin-2- one.
To a stirred solution of optically enriched (4R,5R)-4-(5-bromopyridin-3-yl)-5- (2,5-difluorophenyl)oxazolidin-2-one (1.25 g, 3.25 mmol) in triethylamine (70 mL) was added ethynylbenzene (0.592 mL, 5.28 mmol), copper(I) iodide (67 mg, 0.352 mmol), and triphenylphosphine (653 mg, 2.464 mmol). Nitrogen was bubbled through the mixture for 10 mintues before adding dichlorobis(triphenylphosphine)- palladium(II) (202 mg, 0.282 mmol) with continued nitrogen gas bubbling. After an additional 10 mintues the reaction mixtrue was heated to reflux for 16 hours, cooled to ambient temperature, diluted with EtOAc, washed with water (3X), brine, dried over magnesium sulfate, and concentrated in vacuo. Column chromatography (25% – -> 40% EtO Ac/Hex) provided optically enriched (4R,5R)-5-(2,5-difluorophenyl)-4- (5-(phenylethynyl)pyridin-3-yl)oxazolidin-2-one which was separated by chiral SFC chromatography (Chiralcel OJ-H preparative column, 30 x 250mm, 5μιη, Mobile Phase: 40% MeOH (0.1%DEA) in C02 @ 150Bar, Temp: 35°C, Flow rate: 70.0 mL/min. for 16 min, UV monitored @ 280 nM . tR = 9.23 min) to provide (1.38 g, 2.99 mmol, 85 % yield) of pure single enantiomer (4R,5R)-5-(2,5-difluorophenyl)- 4-(5-(phenylethynyl)pyridin-3-yl)oxazolidin-2-one.
‘H NMR (500 MHz, DMSO-i¾) δ ppm 8.77 (d, J=2.21 Hz, 1 H) 8.57 (s, 1 H) 8.56 (d, J=2.20 Hz, 1 H) 8.07 (t, J=2.05 Hz, 1 H) 7.58 – 7.66 (m, 2 H) 7.44 – 7.52 (m, 3 H) 7.39 – 7.45 (m, 1 H) 7.28 – 7.39 (m, 2 H) 5.67 (d, J=6.62 Hz, 1 H) 5.04 (d, J=6.62 Hz, 1 H). 13C NMR (126 MHz,
DMSO-i¾) δ ppm 157.28; 157.24 (d, J=240.70 Hz) 155.92 (d, J=245.20 Hz) 151.63; 147.70; 136.78; 135.02; 131.57; 129.43; 128.89; 126.63 (dd, J=14.99, 7.72 Hz) 121.51; 119.47; 117.83 (dd, J=23.60, 9.10 Hz) 117.50 (dd, J=24.50, 8.20 Hz); 114.60 (dd, J=26.34, 4.54 Hz); 92.86; 85.76; 78.12; 59.43;
LCMS (ESI) m/z calcd for C22H15F2N202: 377.11, found 377.20[M+H]+;
HRMS (ESI) m/z calcd for
C22H15F2N202: 377.1096, found 377.1096 [M+H]+.
SEE
WO2015054103, OXAZOLIDINONES AS MODULATORS OF MGLUR5
PAPER
Clik here to view.

Positive allosteric modulators (PAMs) of the metabotropic glutamate receptor subtype 5 (mGluR5) are of interest due to their potential therapeutic utility in schizophrenia and other cognitive disorders. Herein we describe the discovery and optimization of a novel oxazolidinone-based chemotype to identify BMS-955829 (4), a compound with high functional PAM potency, excellent mGluR5 binding affinity, low glutamate fold shift, and high selectivity for the mGluR5 subtype. The low fold shift and absence of agonist activity proved critical in the identification of a molecule with an acceptable preclinical safety profile. Despite its low fold shift, 4 retained efficacy in set shifting and novel object recognition models in rodents.
Discovery and Preclinical Evaluation of BMS-955829, a Potent Positive Allosteric Modulator of mGluR5
http://pubs.acs.org/doi/abs/10.1021/acsmedchemlett.5b00450
http://pubs.acs.org/doi/suppl/10.1021/acsmedchemlett.5b00450/suppl_file/ml5b00450_si_001.pdf
SEE…………http://orgspectroscopyint.blogspot.in/2016/01/bms-955829.html
///////BMS 955829, mGluR5, positive allosteric modulator, schizophrenia, cognition, neurotoxicity, Bristol-Myers Squibb
FC1=CC=C(C=C1[C@H]([C@@H](C2=CC(C#CC3=CC=CC=C3)=CN=C2)N4)OC4=O)F
Filed under: Preclinical drugs Tagged: BMS 955829, Bristol-Myers Squibb, cognition, mGluR5, neurotoxicity, positive allosteric modulator, schizophrenia Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.






















