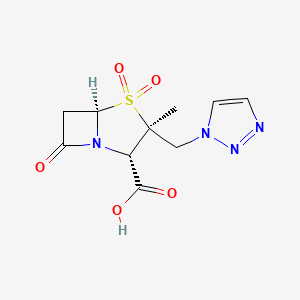
Tazobactam; Tazobactam acid; 89786-04-9; Tazobactamum; CHEMBL404; YTR-830H;
(2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
| Molecular Formula: | C10H12N4O5S |
|---|---|
| Molecular Weight: | 300.29108 g/mol |
Tazobactam is a beta Lactamase Inhibitor. The mechanism of action of tazobactam is as a beta Lactamase Inhibitor.
Tazobactam is a penicillanic acid sulfone derivative and beta-lactamase inhibitor with antibacterial activity. Tazobactam contains a beta-lactam ring and irreversibly binds to beta-lactamase at or near its active site. This protects other beta-lactam antibiotics from beta-lactamase catalysis. This drug is used in conjunction with beta-lactamase susceptible penicillins to treat infections caused by beta-lactamase producing organisms.
Tazobactam is a pharmaceutical drug that inhibits the action of bacterial β-lactamases, especially those belonging to the SHV-1 and TEM groups. It is commonly used as its sodium salt, tazobactam sodium.
Tazobactam is combined with the extended spectrum β-lactam antibiotic piperacillin in the drug piperacillin/tazobactam, one of the preferred antibiotic treatments for nosocomial pneumonia caused by Pseudomonas aeruginosa.[citation needed] Tazobactam broadens the spectrum of piperacillin by making it effective against organisms that express β-lactamase and would normally degrade piperacillin.[1]
Tazobactam is a heavily modified penicillin and a sulfone.
synthesis coming……………
References
- Yang Y, Rasmussen BA, Shlaes DM (1999). “Class A beta-lactamases—enzyme-inhibitor interactions and resistance”. Pharmacol Ther. 83: 141–151. doi:10.1016/S0163-7258(99)00027-3.
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
(2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide
|
|
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Intravenous |
| Identifiers | |
| CAS Number | 89786-04-9  |
| ATC code | J01CG02 |
| PubChem | CID: 123630 |
| DrugBank | DB01606  |
| ChemSpider | 110216  |
| UNII | SE10G96M8W  |
| KEGG | D00660  |
| ChEBI | CHEBI:9421  |
| ChEMBL | CHEMBL404  |
| Chemical data | |
| Formula | C10H12N4O5S |
| Molecular mass | 300.289 g/mol |
| Patent | Submitted | Granted |
|---|---|---|
| 2-OXO-1-AZETIDINE SULFONIC ACID DERIVATIVES AS POTENT BETA-LACTAMASE INHIBITORS [EP0979229] | 2000-02-16 | 2002-10-23 |
| DHA-pharmaceutical agent conjugates of taxanes [US7199151] | 2004-09-16 | 2007-04-03 |
| Antimicrobial composition comprising a vinyyl pyrrolidinon derivative and a carbapenem antibiotic or a beta-lactamase inhibitor [EP0911030] | 1999-04-28 | 2005-04-13 |
| 7-alkylidene-3-substituted-3-cephem-4-carboxylates as beta-lactamase inhibitors [US7488724] | 2006-04-06 | 2009-02-10 |
| Sustained release of antiinfectives [US7718189] | 2006-04-06 | 2010-05-18 |
| Conjugate of fine porous particles with polymer molecules and the utilization thereof [US2006159715] | 2006-07-20 | |
| ENGINEERED BACTERIOPHAGES AS ADJUVANTS FOR ANTIMICROBIAL AGENTS AND COMPOSITIONS AND METHODS OF USE THEREOF [US2010322903] | 2009-01-12 | 2010-12-23 |
| Microparticles for the treatment of disease [US2010323019] | 2010-08-19 | 2010-12-23 |
| Packaging System [US2010326868] | 2010-08-30 | 2010-12-30 |
| COMBINATION ANTIBIOTIC AND ANTIBODY THERAPY FOR THE TREATMENT OF PSEUDOMONAS AERUGINOSA INFECTION [US2010272736] | 2010-02-04 | 2010-10-28 |
/////////
O=S2(=O)[C@]([C@@H](N1C(=O)C[C@H]12)C(=O)O)(Cn3nncc3)C
or
CC1(C(N2C(S1(=O)=O)CC2=O)C(=O)O)CN3C=CN=N3
Filed under: Uncategorized Tagged: tazobactam