
Semapimod Mesylate
CPSI-2364, AXD-455, CN-1493, CNI 1493
CAS No. 352513-83-8(Semapimod)
…………..(Semapimod mesylate)
MW 1129
CROHNS DISEASE, PHASE 1
N,N’-bis[3,5-bis[(E)-N-(diaminomethylideneamino)-C-methylcarbonimidoyl]phenyl]decanediamide
N,N’-Bis(3,5-bis(1-(carbamimidoylhydrazono)ethyl)phenyl)decanediamide
- N,N’-Bis(3-acetylphenyl)decane diamide tetrakis (amidinohydrazone)
- N,N’-bis(3,5-bis{(1E)-N-[amino(imino)methyl]ethanehydrazonoyl}phenyl)decanediamide
- N,N’-bis[3,5-bis[(E)-N-(diaminomethylideneamino)-C-methylcarbonimidoyl]phenyl]decanediamide
- N,N’-bis[3,5-bis[(E)-N-guanidino-C-methyl-carbonimidoyl]phenyl]decanediamide
A nitric oxide synthesis inhibitor and a p38 MAPK inhibitor potentially for the treatment of Crohn’s disease.

Crohn’s disease (CD) is a chronic inflammatory disease involving the upper and lower gastrointestinal tract and characterized by abdominal pain, weight loss, gastrointestinal bleeding and formation of fistulas between loops of bowel and from the bowel to the skin or other organs. Current therapy for active Crohn’s disease consists of symptomatic treatment, nutritional therapy, salicylates and immunosuppressants or surgical management.
Tumor necrosis factor a (TNF-a) plays a central role in the initiation and amplification of the granulomatous inflammatory reaction seen in CD (van Deventer, 1997). Increased TNF-a is present in gut mucosa as well as in stool of patients with active CD (Braegger et al, 1992). CNI-1493 is a synthetic guanylhydrazone compound that is an inhibitor of TNF-a synthesis. A monoclonal antibody to TNF, infliximab, is now approved for treatment of CD, but not all patients respond and many who do respond eventually become refractory to this treatment as well.
CNI-1493 is a synthetic compound which blocks the production of several inflammatory cytokines, including TNF. Because it blocks production of multiple inflammatory mediators, it may be more active than products targeted to a specific cytokine. In addition, as it is not a biologic, it should not cause hypersensitivity reactions or induce formation of antibodies.
The purpose of this trial is to determine if CNI-1493 is safe and effective in treating patients with moderate to severe Crohn’s Disease in a placebo controlled setting………https://clinicaltrials.gov/ct2/show/NCT00038766
Semapimod (INN), formerly known as CNI-1493, is an investigational new drug which has anti-inflammatory,[1] anti-cytokine,[2] immunomodulatory,[3] antiviral[4] and antimalarial[5] properties.
History
Semapimod was developed at the former Picower Institute for Medical Research, and is now licensed to Cytokine PharmaSciences. In 2000, Cytokine PharmaSciences licensed anti-infective applications of semapimod to Axxima Pharmaceuticals, but Axxima became insolvent in Dec. 2004 and its assets were acquired by GPC Biotech, which has recently merged into Agennix AG[1]. Although the disposition of Axxima’s partial rights to semapimod was not specified in these merger announcements, Cytokine PharmaSciences does not currently list any licensees for semapimod on its website.
Mechanism of action
Semapimod was first developed to inhibit nitric oxide synthesis by inflammatory macrophages, via inhibition of the uptake of arginine which macrophages require for nitric oxide synthesis.[1] Subsequently it was found that suppression of nitric oxide synthesis occurred even at semapimod concentrations 10-fold less than required for inhibition of arginine uptake, suggesting that this molecule was a more general inhibitor of inflammatory responses.[2] Further work revealed that semapimod suppressed the translation efficiency of tumor necrosis factor production.[6] Specifically, semapimod was found to be an inhibitor of p38 MAP kinase activation.[7] Surprisingly, however, the primary mode of action in vivo is now thought to be via stimulation of the vagus nerve, thereby down-regulating inflammatory pathways via the recently discovered cholinergic anti-inflammatory pathway.[8][9]
Pharmacology and clinical trials
In a preclinical study in rats, semapimod was found to suppress cytokine-storm induction by the anticancer cytokine interleukin-2 (IL-2) without decreasing its anticancer properties, allow larger doses of IL-2 to be administered.[10] A subsequent phase I trial in humans failed to show an increase in the tolerated dose of IL-2, although indications of pharmacological activity as an inhibitor of tumor necrosis factor production were observed.[11]
In a preliminary clinical trial of semapimod in patients with moderate to severe Crohn’s disease, positive clinical changes were observed, including endoscopic improvement, positive responses in some patients not responding to infliximab, healing of fistulae, and indications for tapering of steroids; no significant adverse effects were observed.[12]
In a small clinical trial against post-ERCP pancreatitis, significant suppression was not observed, although investigators observed a significant reduction of the incidence of hyperamylasemia and the levels of post-ERCP amylase.[13]
In the clinical trials above, semapimod tetrahydrochloride was administered by intravenous injection. This route has drawbacks such as dose-limiting phlebitis.[2] Recently Cytokine PharmaSciences has announced the development of novel salt forms of semapimod which are said to be orally absorbable; a phase I clinical trial of one of these salt forms, CPSI-2364, has been completed, and a phase II trial is planned for 2010.[3][4]
Chemistry
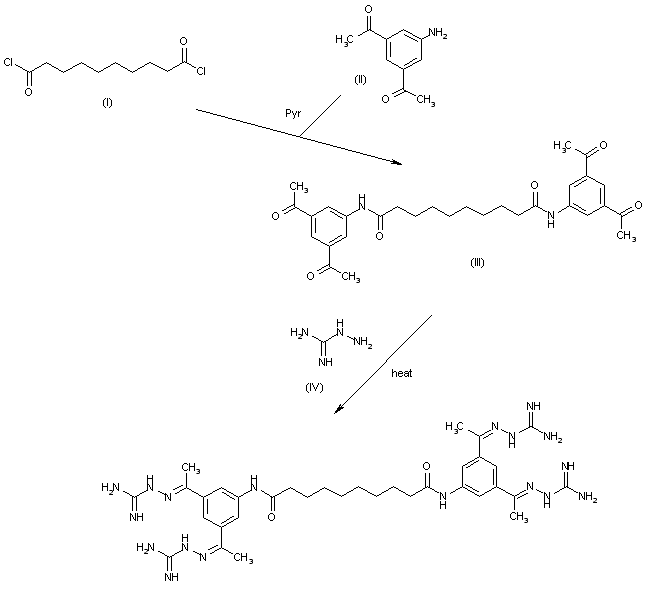
Semapimod is synthesized by reacting 3,5-diacetylaniline[14] with sebacoyl chloride in the presence of pyridine, followed by reaction of the resulting tetraketone with aminoguanidine hydrochloride.[1]
PATENT
-
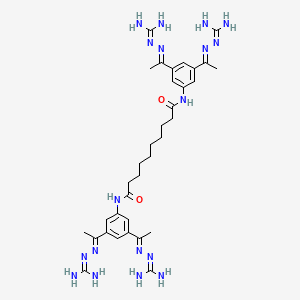
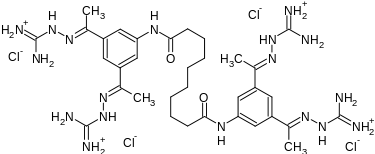
N,N′-bis(3,5-diacetylphenyl) decanediamide tetrakis (amidinohydrazone) tetrahydrochloride (CNI-1493), which has the following structural formula:
SYNTHESIS
The reaction of decanedioyl dichloride (I) with 3,5-diacetylaniline (II) by means of pyridine in dichloromethane gives the corresponding diamide (III), which is condensed with aminoguanidine (IV) in refluxing aqueous ethanol to afford the target tetrakis amidinohydrazone. EP 0746312; EP 1160240; US 5599984; WO 9519767
http://www.google.com/patents/EP0746312A1?cl=en
References
- 1 Bianchi, M.; Ulrich, P.; Bloom, O.; Meistrell m, M. , I. I.; Zimmerman, G. A.; Schmidtmayerova, H.; Bukrinsky, M.; Donnelley, T.; Bucala, R.; Sherry, B.; Manogue, K. R.; Tortolani, A. J.; Cerami, A.; Tracey, K. J. (Mar 1995). “An inhibitor of macrophage arginine transport and nitric oxide production (CNI-1493) prevents acute inflammation and endotoxin lethality”. Molecular Medicine (Cambridge, Mass.) 1 (3): 254–266. ISSN 1076-1551. PMC 2229913. PMID 8529104.
- 2
- Bianchi, M.; Bloom, O.; Raabe, T.; Cohen, P. S.; Chesney, J.; Sherry, B.; Schmidtmayerova, H.; Calandra, T.; Zhang, X.; Bukrinsky, M.; Ulrich, P.; Cerami, A.; Tracey, K. J. (Mar 1996). “Suppression of proinflammatory cytokines in monocytes by a tetravalent guanylhydrazone”. The Journal of Experimental Medicine 183 (3): 927–936. doi:10.1084/jem.183.3.927. ISSN 0022-1007. PMC 2192362. PMID 8642296.
- 3
- Martiney, J.; Rajan, A. J.; Charles, P. C.; Cerami, A.; Ulrich, P. C.; MacPhail, S.; Tracey, K. J.; Brosnan, C. F. (Jun 1998). “Prevention and treatment of experimental autoimmune encephalomyelitis by CNI-1493, a macrophage-deactivating agent” (Free full text). Journal of immunology (Baltimore, Md. : 1950) 160 (11): 5588–5595. ISSN 0022-1767. PMID 9605164.
- 4
- Hauber, I.; Bevec, D.; Heukeshoven, J.; Krätzer, F.; Horn, F.; Choidas, A.; Harrer, T.; Hauber, J. (Jan 2005). “Identification of cellular deoxyhypusine synthase as a novel target for antiretroviral therapy”. The Journal of Clinical Investigation (Free full text) 115 (1): 76–85. doi:10.1172/JCI21949. ISSN 0021-9738. PMC 539192. PMID 15630446.
- 5
- Specht, S.; Sarite, R.; Hauber, I.; Hauber, J.; Görbig, F.; Meier, C.; Bevec, D.; Hoerauf, A.; Kaiser, A. (May 2008). “The guanylhydrazone CNI-1493: an inhibitor with dual activity against malaria-inhibition of host cell pro-inflammatory cytokine release and parasitic deoxyhypusine synthase”. Parasitology research 102 (6): 1177–1184. doi:10.1007/s00436-008-0891-x. ISSN 0932-0113. PMID 18256853.
- 6
- Cohen, P. S.; Nakshatri, H.; Dennis, J.; Caragine, T.; Bianchi, M.; Cerami, A.; Tracey, K. J. (Apr 1996). “CNI-1493 inhibits monocyte/macrophage tumor necrosis factor by suppression of translation efficiency”. Proceedings of the National Academy of Sciences of the United States of America 93 (9): 3967–3971. Bibcode:1996PNAS…93.3967C. doi:10.1073/pnas.93.9.3967. ISSN 0027-8424. PMC 39469. PMID 8632999.
- 7
- Cohen, P. S.; Schmidtmayerova, H.; Dennis, J.; Dubrovsky, L.; Sherry, B.; Wang, H.; Bukrinsky, M.; Tracey, K. J. (May 1997). “The critical role of p38 MAP kinase in T cell HIV-1 replication”. Molecular Medicine (Cambridge, Mass.) 3 (5): 339–346. ISSN 1076-1551. PMC 2230081. PMID 9205949.
- 8
- Tracey, J. (Feb 2007). “Physiology and immunology of the cholinergic antiinflammatory pathway”. The Journal of Clinical Investigation (Free full text) 117 (2): 289–296. doi:10.1172/JCI30555. ISSN 0021-9738. PMC 1783813. PMID 17273548.
- 9
- Oke, L.; Tracey, J. (Mar 2008). “From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex” (Free full text). Journal of Leukocyte Biology 83 (3): 512–517. doi:10.1189/jlb.0607363. ISSN 0741-5400. PMID 18065685.
- 10
- Kemeny, M. M.; Botchkina, G. I.; Ochani, M.; Bianchi, M.; Urmacher, C.; Tracey, K. J. (1998). “The tetravalent guanylhydrazone CNI-1493 blocks the toxic effects of interleukin-2 without diminishing antitumor efficacy”. Proceedings of the National Academy of Sciences of the United States of America 95 (8): 4561–4566. Bibcode:1998PNAS…95.4561K. doi:10.1073/pnas.95.8.4561. PMC 22529. PMID 9539777.
- 11
- Atkins, M. B.; Redman, B.; Mier, J.; Gollob, J.; Weber, J.; Sosman, J.; MacPherson, B. L.; Plasse, T. (2001). “A phase I study of CNI-1493, an inhibitor of cytokine release, in combination with high-dose interleukin-2 in patients with renal cancer and melanoma”. Clinical Cancer Research 7 (3): 486–492. PMID 11297238.
- 12
- Hommes, D.; Van Den Blink, B.; Plasse, T.; Bartelsman, J.; Xu, C.; MacPherson, B.; Tytgat, G.; Peppelenbosch, M.; Van Deventer, S. (2002). “Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease”. Gastroenterology 122 (1): 7–14. doi:10.1053/gast.2002.30770. PMID 11781274.
- 13 Vanwesterloo, D.; Rauws, E.; Hommes, D.; De Vos, A.; Van Der Poll, T.; Powers, B.; Fockens, P.; Dijkgraaf, M.; Bruno, M. (2008). “Pre-ERCP infusion of semapimod, a mitogen-activated protein kinases inhibitor, lowers post-ERCP hyperamylasemia but not pancreatitis incidence”. Gastrointestinal Endoscopy 68 (2): 246–254. doi:10.1016/j.gie.2008.01.034. PMID 18455169.
- 14 Ulrich, P.; Cerami, A. (Jan 1984). “Trypanocidal 1,3-arylene diketone bis(guanylhydrazone)s. Structure-activity relationships among substituted and heterocyclic analogues”. Journal of Medical Chemistry 27 (1): 35–40. doi:10.1021/jm00367a007. ISSN 0022-2623. PMID 6690682.
| Patent | Submitted | Granted |
|---|---|---|
| Neural tourniquet [US2005282906] | 2005-12-22 | |
| Guanylhydrazone Salts, Compositions, Processes of Making, and Methods of Using [US2008262090] | 2008-10-23 | |
| Protective role of semapimod in necrotizing enterocolitis [US7795314] | 2007-12-06 | 2010-09-14 |
| METHOD OF TREATING ILEUS BY PHARMACOLOGICAL ACTIVATION OF CHOLINERGIC RECEPTORS [US2011112128] | 2011-05-12 | |
| Method of treating ileus by pharmacological activation of cholinergic receptors [US2007213350] | 2007-09-13 | |
| Pharmaceutically active aromatic guanylhydrazones [US2005171176] | 2005-08-04 | |
| Guanylhydrazone salts, compositions, processes of making and methods of using [US7244765] | 2006-01-19 | 2007-07-17 |
| GUANYLHYDRAZONE SALTS, COMPOSITIONS, PROCESSES OF MAKING, AND METHODS OF USING [US8034840] | 2008-06-19 | 2011-10-11 |
| METHOD FOR TREATING GLIOBLASTOMAS AND OTHER TUMORS [US2014323576] | 2014-03-14 | 2014-10-30 |
| Methods of treatment of fatty liver disease by pharmacological activation of cholinergic pathways [US8865641] | 2012-06-14 | 2014-10-21 |
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
N,N’-bis[3,5-bis[N-(diaminomethylideneamino)-C-methylcarbonimidoyl]phenyl] decanediamide tetrahydrochloride
|
|
| Identifiers | |
| CAS Number | 164301-51-3  352513-83-8 (base) |
| ATC code | None |
| PubChem | CID: 5745214 |
| UNII | 9SGW2H1K8P  |
| ChEMBL | CHEMBL2107779 |
| Chemical data | |
| Formula | C34H56Cl4N18O2 |
| Molecular mass | 890.73984 g/mol |
/////////Semapimod Mesylate, CPSI-2364, AXD-455, CN-149, PHASE 1, FERRING, CNI 1493
CC(=NN=C(N)N)C1=CC(=CC(=C1)NC(=O)CCCCCCCCC(=O)NC2=CC(=CC(=C2)C(=NN=C(N)N)C)C(=NN=C(N)N)C)C(=NN=C(N)N)C
Filed under: PHASE1 Tagged: AXD-455, CN-149, CNI 1493, CPSI-2364, FERRING, PHASE 1, Semapimod Mesylate