Zucapsaicin (珠卡赛辛)
cis-Capsaicin; (Z)-Capsaicin
Zucapsaicin; Civamide; Cis-Capsaicin; 25775-90-0; (Z)-Capsaicin; (Z)-N-(4-Hydroxy-3-methoxybenzyl)-8-methylnon-6-enamide;
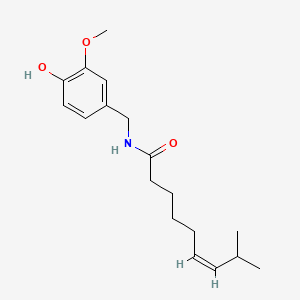
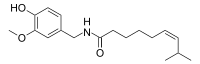
(Z)-N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
CAS No. 25775-90-0
| MF C18H27NO3 | |
| Molecular Weight: | 305.41188 g/mol |
|---|
WINSTON INNOVATOR
SANOFI
(Zuacta®/Civanex®
A medication used to treat osteoarthritis of the knee and other neuropathic pain.TRPV1 CHANNEL AGONIST


Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. It is applied three times daily for a maximum of three months. It reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use (patch and cream).[1]
Zucapsaicin has been tested for treatment of a variety of conditions associated with ongoing nerve pain. This includes herpes simplex infections; cluster headaches and migraine; and knee osteoarthritis.[2]
Civanex (zucapsaicin) cream is a TRPV-1 modulator in development for the treatment of signs and symptoms of osteoarthritis of the knee.
Zucapsaicin, the cis-isomer of the natural product capsaicin, is a
topical analgesic that was initially developed by Winston Pharmaceuticals
and approved in Canada in July 2010 for the treatment of
severe pain in adults with osteoarthritis of the knee.
Bronson, J.; Dhar, M.; Ewing, W.; Lonberg, N. In Annual Reports in MedicinalChemistry; John, E. M., Ed.; Academic Press, 2011; Vol. 46, p 433.
The advantagesof zucapsaicin compared with naturally-occurring capsaicin, are reported to be a lesser degree of local irritation (stinging, burning,
erythema) in patients and a greater degree of efficacy in preclinical
animal models of pain.
Bernstein, J. E. U.S. 5063060, 1991.
Bernstein, J. E. U.S. 20050084520 A1, 2005.
The analgesic action of both
zucapsaicin and capsaicin is mediated through the transient receptor
potential vanilloid type 1 (TRPV1) channel, a ligand-gated ion
channel expressed in the spinal cord, brain, and localized on neurons
in sensory projections to the skin, muscles, joints, and
gut.
Westaway, S. M. J. Med. Chem. 2007, 50, 2589.
The scale preparation of zucapsaicin likely parallels the original
approach described by Gannett and co-workers involving the
coupling of vanillylamine with (Z)-8-methylnon-6-enoyl chloride.
Gannett, P. M.; Nagel, D. L.; Reilly, P. J.; Lawson, T.; Sharpe, J.; Toth, B. J. Org.Chem. 1988, 53, 1064.
Orito and co-workers elaborated this original approach in
an effort to prepare both capsaicin and zucapsaicin on gram-scale,
Kaga, H.; Miura, M.; Orito, K. J. Org. Chem. 1989, 54, 3477.
References
- 1 Winston Pharmaceuticals website http://www.winstonlabs.com/productdevelopment/civamide.asp
- 2 Zucapsaicin information from the National Library of Medicine http://druginfo.nlm.nih.gov/drugportal
Janusz, John M.; Buckwalter, Brian L.; Young, Patricia A.; LaHann, Thomas R.; Farmer, Ralph W.; et al. Journal of Medicinal Chemistry, 1993 , vol. 36, # 18 p. 2595 – 2604
Journal of Organic Chemistry, , vol. 53, # 5 p. 1064 – 1071
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
(Z)-N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide
|
|
| Clinical data | |
| Trade names | Civanex |
| Routes of administration |
Topical |
| Identifiers | |
| CAS Number | 25775-90-0 |
| ATC code | M02AB02 |
| PubChem | CID: 1548942 |
| ChemSpider | 1265956 |
| UNII | 15OX67P384 |
| Synonyms | Civamide; (Z)-Capsaicin; cis-Capsaicin |
| Chemical data | |
| Formula | C18H27NO3 |
| Molecular mass | 305.41188 g/mol |
////Zucapsaicin
Oc1ccc(cc1OC)CNC(CCCC\C=C/C(C)C)=O
Filed under: Uncategorized Tagged: Zucapsaicin