Atagabalin
Trans-dimethyl gababutin; UNII-JT7957Q2FB; 223445-75-8;
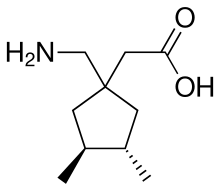
2-[(3S,4S)-1-(aminomethyl)-3,4-dimethylcyclopentyl]acetic acid
2-[(3S,4S)-1-(aminomethyl)-3,4-dimethyl-cyclopentyl]acetic acid
3,4-trans-2-(1-(aminomethyl)-3,4-dimethylcyclopentyl)acetic acid
Cyclopentaneaceticacid, 1-(aminomethyl)-3,4-dimethyl-, (3S,4S)-
Pfizer Inc. INNOVATOR
Atagabalin (PD-0200,390) is a drug developed by Pfizer and related to gabapentin, which similarly binds to the α2δ calcium channels (1 and 2).[1] It was under development as a treatment for insomnia,[2][3][4] but was discontinued following unsatisfactory trial results.
Gabapentin (Neurontin®) (1) was launched as an add-on therapy for epilepsy in 1994. Utility against neuropathic pain and anxiety have been reported preclinically and efficacy against neuropathic pain has been demonstrated clinically in humans. Pregabalin (Lyrica®) (2), has superior potency and pharmacokinetics to gabapentin and has been approved for the management of neuropathic pain associated with diabetic peripheral neuropathy, post-herpetic neuralgia, adjunctive treatment of partial seizures, and fibromyalgia in the US.
Gabapentin and pregabalin are thought to mediate their pharmacological actions through binding to the α2δ subunit of a voltage gated calcium channeland it has been shown that gabapentin and pregabalin bind to this α2δ subunit with IC50 values of 140 nM and 80 nM, respectively. We have recently disclosed our initial SAR investigations around five-membered ring gabapentin analogues, which we have termed gababutins.In that Letter, we investigated a range of 3-substituted gababutin analogues and identified the 3-(R)-methyl gababutins (3) and (4). Both (3) and (4) bind to the gabapentin binding site with high affinity but have different in vivo profiles, with (3) being effective on oral dosing in models of anxiety and (4) being effective on oral dosing in models of neuropathic pain.
SYNTHESIS
PATENT
WO 1999021824
http://www.google.co.in/patents/WO1999021824A1?cl=en
synthesis of 3-oxo-2,8-diazaspiro[4,5]decane-
8-carboxylic acid tert-butyl ester (P. W. Smith et al., J. Med. Chem., 1995;38:3772). The compounds may also be synthesized by the methods outlined by G. Satzinger et al., (Ger Offen 2,460,891; US 4,024,175, and Ger Offen 2,611,690; US 4,152,326) (General Schemes 3 and 4). The compounds may also be synthesized by the route outlined by G. Griffiths et al., Helv. Chim. Ada, 1991 ;74:309 (General Scheme 5). General Scheme 1
(i) Ethyl cyanoacetate, piperidine (Cope et al., J. Am. Chem. S c.,1941 ;63:3452); (ii) NaCN, EtOH/H2O; (iii) EtOH, HCl; (iv) H2O/H+; (v) H2, Rh/C, MeOH; (vi) HCl.
General Scheme 2
(i) Ph3P=CHCO2Me; (ii) MeNO2, 1,1,3,3-tetramethylguanidine; (iii) Raney nickel, EtOH/H2O; (iv) HCl.
General Scheme 3
(i) Ethylcyanoacetate, ammonia then H3θ+; (ii) H2SO4; (iii) AC2O; (iv) MeOH; (v) Curtius Reaction; (vi) HCl, H2O then anion exchange.
General Scheme 4
(i) Ethylcyanoacetate, ammonia then H3O “; (ii) H2SO4; (iii) AC2O; (iv) H2NOH; (v) PhSO2Cl; (vi) Et3N, MeOH; (vii) HCl, H O then anion exchange.
General Scheme 5
(i) Ethyl cyanoacetate, piperidine (Cope et al., J. Am. Chem. Soc, 1941 ;63:3452); (ii) NaCN, EtOH/H2O; (iii) BnOH, HCl; (iv) H2O/H+; (v) H2, Rh/C, MeOH.
EXAMPLE 1
Reagents: (i) Triethylphosphonoacetate, NaH; (ii) MeNO2,Bu4N+F”; (iϋ) H2, Ni; (iv) HCl Synthesis of (trans)-(3,4-Dimethyl-cyclopentylidene)-acetic acid ethyl ester (2)
NaH (60% dispersion in oil, 737 mg, 18.42 mmol) was suspended in dry tetrahydrofuran (50 mL) and cooled to 0°C. Triethylphosphonoacetate (3.83 mL, 19.30 mmol) was added and the mixture stirred at 0°C for 15 minutes. The ketone (1) (1.965 g, 17.54 mmol) in THF (10 mL) was then added and the mixture allowed to warm to room temperature. After 2 hours, the mixture was partitioned between diethyl ether (200 mL) and water (150 mL). The organic phase was separated, washed with brine, dried (MgSO4) and the solvent removed in vacuo.
The residue was purified by flash chromatography (silica, ethyl acetate:heptane 1 :9) to give 3.01 g (94%) of (2) as a colorless oil.
*H NMR 400 MHz (CDCI3): δ 1.01 (3H, d, J = 6 Hz), 1.03 (3H, d, J = 6 Hz), 1.26
(3H, t, J = 7 Hz), 1.49 (2H, m), 2.07 (1H, m), 2.24 (1H, m), 2.61 (1H, m), 4.13 (2H, q, J = 7 Hz), 5.72 (1H, s).
MS (CI+) m/e: 183 ([MH+], 18%).
Synthesis of (trans)-(3,4-Dimethyl-l-nitromethyl-cyclopentyl)-acetic acid ethyl ester (3)
The unsaturated ester (2) (2.95 g, 16.2 mmol) was dissolved in tetrahydrofuran (10 mL) and stirred at 70°C with nitromethane (1.9 mL, 35.2 mmol) and tetrabutylammonium fluoride (1.0 M in tetrahydrofuran, 22 mL, 22.0 mmol). After 6 hours, the mixture was cooled to room temperature, diluted with ethyl acetate (50 mL), and washed with 2N HCl (30 mL) followed by brine (50 mL). The organic phase was collected, dried (MgSO4) and the solvent removed in vacuo. The residue was purified by flash chromatography (silica, ethyl acetate :heptane 1 :9) to give 1.152 g (29%) of a clear oil. !H NMR 400 MHz (CDCI3): δ 0.98 (6H, d, J = 6 Hz), 1.10-1.39 (5H, m), 1.47
(2H, m), 1.87 (1H, m), 2.03 (1H, m), 2.57 (2H, ABq, J = 16, 38 Hz), 4.14 (2H, q, J = 7 Hz), 4.61 (2H, ABq, J = 12, 60 Hz).
MS (ES+) m/e: 244 ([MH+], 8%).
IR (film) v ein-1 : 1186, 1376, 1549, 1732, 2956. Synthesis of (±)-(trans)-7,8-Dimethyl-spiro[4.4]nonan-2-one (4)
The nitroester (3) (1.14 g, 4.7 mmol) was dissolved in methanol (50 mL) and shaken over Raney nickel catalyst under an atmosphere of hydrogen (40 psi) at 30°C. After 5 hours, the catalyst was removed by filtration through celite. The solvent was removed in vacuo to give 746 mg (95%) of a pale yellow oil which solidified on standing.
! H NMR 400 MHz (CDC13): δ 0.98 (6H, d, J = 6 Hz), 1.32 (2H, m), 1.46 (2H, m), 1.97 (2H, m), 2.27 (2H, ABq, J = 16, 27 Hz), 3.23 (2H, s), 5.62 (1H, br s). MS (ES+) m/e: 168 ([MH+], 100%). IR Cfilπ v cm-1 : 1451, 1681, 1715, 2948, 3196.
Synthesis of (±)-(trans)-(l-Aminomethyl-3,4-dimethyl-cyclopentyl)-acetic acid hydrochloride (5)
The lactam (4) (734 mg, 4.40 mmol) was heated to reflux in a mixture of 1 ,4-dioxan (5 mL) and 6N HCl (15 mL). After 4 hours, the mixture was cooled to room temperature, diluted with water (20 mL), and washed with dichloromethane
(3 x 30 mL). The aqueous phase was collected and the solvent removed in vacuo. The residue was triturated with ethyl acetate to give 675 mg (69%) of a white solid after collection and drying.
ΪH NMR 400 MHz (d6-DMSO): δ 0.91 (6H, d, J = 6 Hz), 1.18 (2H, m), 1.42 (2H, m), 1.72 (1H, m), 1.87 (1H, m), 2.42 (2H, ABq, J = 16, 24Hz), 2.90 (2H, ABq,
J = 12, 34 Hz), 8.00 (3H, br s), 12.34 (1H, br s).
MS (ES+) m/e: 186 ([MH-HC1J+, 100%).
PATENT
WO 2002000209
PATENT
http://www.google.co.in/patents/WO1999021824A1?cl=en
PATENT
WO 2007010387
http://www.google.com/patents/WO2007010387A2?cl=en
21 22
Scheme IH
PAPER
Synthesis and in vivo evaluation of 3,4-disubstituted gababutins
Bioorganic&Medicinal Chemistry Letters (2010), 20, (1), 248-251.
The synthesis of 3,4-trans-dimethyl cyclopentanone (14), is detailed in Scheme 1.
![Reagents and conditions: (i) (−)-menthol, pyridine, CH2Cl2; (ii) butadiene, ...]()
-
Scheme 1.
Reagents and conditions: (i) (−)-menthol, pyridine, CH2Cl2; (ii) butadiene, TiCl4, toluene, −10 °C (100% yield, 65% de) or butadiene, Et2AlCl, toluene, −60 °C (64% yield, 95% de); (iii) LiAlH4, THF; recrystallisation from acetone; (iv) pyridine, MsCl, 0 °C, 18h (82%); (v) LiAlH4, diethyl ether, 40 °C, 2h (98%); (vi) KMnO4, nBu4NBr, H2O–CH2Cl2, rt, 18h; then SO2, 0 °C (82%); (vii) methanol, cH2SO4, rt, 18h (90%) (viii) KOtBu, THF, 75 °C, 3h (100%); (ix) DMSO, H2O, 140 °C, 4 h (86%).
![Reagents and conditions: (i) triethylphosphonoacetate, NaH, THF, 0°C to rt ...]()
-
Scheme 3.
Reagents and conditions: (i) triethylphosphonoacetate, NaH, THF, 0 °C to rt (95%); (ii) MeNO2, TBAF, THF, reflux (65%); (iii) H2, Ni, MeOH; (iv) 6 N HCl, 1,4-dioxane, reflux (69% from nitroester).
References
- 2 Corrigan B, Feltner DE, Ouellet D, Werth JL, Moton AE, Gibson G (August 2009). “Effect of renal impairment on the pharmacokinetics of PD 0200390, a novel ligand for the voltage-gated calcium channel alpha-2-delta subunit”. British Journal of Clinical Pharmacology 68 (2): 174–80. doi:10.1111/j.1365-2125.2009.03444.x. PMC 2767279. PMID 19694735.
- 3 Quintero JE, Pomerleau F, Huettl P, Johnson KW, Offord J, Gerhardt GA (May 2011). “Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: Basic characterization and drug pharmacology”. Brain Research 1401: 1–9. doi:10.1016/j.brainres.2011.05.025. PMID 21664606.
- 4 Kjellsson MC, Ouellet D, Corrigan B, Karlsson MO (June 2011). “Modeling Sleep Data for a New Drug in Development using Markov Mixed-Effects Models”. Pharmaceutical Research 28 (10): 2610–27. doi:10.1007/s11095-011-0490-x. PMID 21681607.
| Patent | Submitted | Granted |
|---|---|---|
| Pyrazolo[4,3-d]pyrimidines as Phosphodiesterase Inhibitors [US7572799] | 2005-11-03 | 2009-08-11 |
| Substituted morpholine compounds for the treatment of central nervous system disorders [US7659394] | 2005-11-03 | 2010-02-09 |
| Therapeutic pyrazolo[3,4-B]pyridines and indazoles [US7423054] | 2006-06-01 | 2008-09-09 |
| Amide derivatives as ion-channel ligands and pharmaceutical compositions and methods of using the same [US7312233] | 2006-09-14 | 2007-12-25 |
| Compounds useful in therapy [US7482375] | 2006-10-26 | 2009-01-27 |
| Therapeutic pyrazolo[3,4-b]pyridines and indazoles [US7485636] | 2006-09-28 | 2009-02-03 |
| Substituted N-sulfonylaminophenylethyl-2-phenoxyacetamide compounds as VR1 receptor antagonists [US7566739] | 2006-09-14 | 2009-07-28 |
| Amide derivatives as ion-channel ligands and pharmaceutical compositions and methods of using the same [US7576099] | 2006-08-31 | 2009-08-18 |
| Substituted sulfonylaminoarylmethyl cyclopropanecarboxamide as VR1 receptor antagonists [US7622589] | 2006-09-21 | 2009-11-24 |
| Alpha 2 Delta Ligands for Fibromyalgia and Other Disorders [US2009203782] | 2009-08-13 |
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
[(3S,4S)-1-(aminomethyl)-3,4-dimethylcyclopentyl]acetic acid
|
|
| Identifiers | |
| CAS Registry Number | 223445-75-8  |
| ATC code | None |
| PubChem | CID: 9794485 |
| ChemSpider | 7970252  |
| UNII | JT7957Q2FB  |
| ChEMBL | CHEMBL593430  |
| Chemical data | |
| Formula | C10H19NO2 |
| Molecular mass | 185.263 g/mol |
//////C[C@H]1CC(C[C@@H]1C)(CC(=O)O)CN
READ IMAGABALIN, PD 217074
Filed under: Uncategorized Tagged: Atagabalin












