 .
.
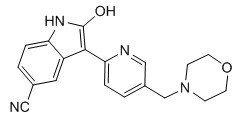
AZD 1080
2-Hydroxy-3-[5-(morpholin-4-ylmethyl)pyridin-2-yl]-1H-indole-5-carbonitrile
2-hydroxy-3-[5-(morpholin-4-ylmethyl)pyridin-2-yl]1H-indole-5-carbonitrile
AZD1080 is a selective, orally active, brain permeable GSK3 inhibitor, inhibits human GSK3α and GSK3β with Ki of 6.9 nM and 31 nM, respectively, shows >14-fold selectivity against CDK2, CDK5, CDK1 and Erk2.
Cas 612487-72-6, AZD1080,
AZD-1080, a glycogen synthase kinase 3 (GSK-3) inhibitor, had been in early clinical trials for the treatment of Alzheimer’s type dementia by AstraZeneca
| Astrazeneca Ab |
PATENTS
WO 2003082853
http://www.google.com/patents/WO2003082853A1?cl=en
PAPER
Organic Process Research & Development (2008), 12(3), 540-543.
http://pubs.acs.org/doi/abs/10.1021/op800020r

A mild and robust method for the large-scale palladium-catalysed cyanation of aryl bromides has been developed. The reaction is sensitive to cyanide poisoning of the catalyst, and it was found that the order of adding the reagents had a strong impact on the performance of the reaction. Addition of the cyanide source to a preheated mixture of the other reagents was critical for achieving a robust and scaleable process. This improved protocol allowed the reaction to be run to full conversion within 3 h at 50 °C on a 6.7 kg scale. Furthermore, it led to the identification of several new efficient catalysts for the reaction.
2-hydroxy-3-[5-(morpholin-4-ylmethyl)pyridin-2-yl]1H-indole-5-carbonitrile (2) (5.2 kg, 15.6 mol), 90% yield with a purity of >90% by HPLC. 1H NMR (d6-DMSO, 400 MHz) δ 14.79 (broad s, 1H), 10.86 (broad s, 1H), 8.08 (s, 1H), 7.95 (s, 1H), 7.83 (d, J = 8.8 Hz, 1H), 7.27 (dd,J = 8.0, 0.9 Hz, 1H), 7.01 (d, J = 8.0 Hz, 1H), 3.57 (t, J = 4.4 Hz, 4H), 3.36 (s, 2H), 2.36 (broad s, 4H); 13C NMR (d6-DMSO, 100 MHz) δ 168.8, 148.6, 141.8, 137.0, 136.1, 125.4, 123.9, 122.3, 121.1, 118.8, 118.3, 108.7, 101.3, 84.6, 66.1, 58.4, 52.8. MS (ES) m/z [M + 1] 335.
PAPER
Topics in Organometallic Chemistry (2012), 42(Organometallics as Catalysts in the Fine Chemical Industry), 125-134.
http://link.springer.com/chapter/10.1007%2F3418_2011_25
PATENT
https://www.google.co.in/patents/WO2007089193A1?cl=en
In the above scheme, preferably Rl is bromo and X is chloro.
Synthesis of 2-Hydroxy-3-[5-(morpholin-4-ylmethyl)pyridin-2-yl] lH-indole-5-carbonitrile citrate
Example 14
2-Hydroxy-3-r5-(moφholin-4-ylmethyl)pyridin-2-yl1 lH-indole-5-carbonitrile citrate salt 2-Hydroxy-3-[5-(moφholin-4-ylmethyl)pyridin-2-yl] lH-indole-5-carbonitrile (5.14 kg, 15.4 mol) was suspended in ethanol (54 L) at room temperature. The suspension was heated to an inner temperature of 700C and a solution of citric acid (3.424 kg, 17.82 mol, 1.300 eq)) in water (103 L) was added keeping the inner temperature above 650C. The mixture was heated to reflux. After this the resulting solution was mixed with activated charcoal (0.412 kg) and reflux continued for 3.5 h after which the reaction mixture was clear filtered at 830C followed by cooling to room temperature over 20 h. After filtration the precipitate was washed twice with a cold mixture of ethanol/water (6.9 L/13.7 L). Drying under vacuum at 5O0C gave 6.648 kg, 82.2% yield of 2-hydroxy-3-[5-(morpholin- 4-ylmethyl)pyridin-2-yl]lH-indole-5-carbonitrile citrate having a purity of at least 98%. The palladium content was less than 1 ppm and the zinc content was lower than 10 ppm. 1H NMR (Jd-DMSO3 400 MHz) δ 14.7 (br s, 1 H), 11.55 (s, 1 H), 10.98 (s, IH), 8.31 (s, 1 H), 8.08 (br d, J= 1.84Hz, IH), 8.02 (s, IH), 7.90 (br d, J = 8.92Hz, 1 H), 7.31 (d, J = 8.0 Hz, 1 H), 7.02 (d, J= 8.0Hz), 4.28 (s, 2 H), 3.97 (m, 2 H), 3.94 (m, 2H), 3.35 (m, 9H), 3.32 (m, 2H) ppm; 13C NMR (d6-DMSO, 400MHz) δ 168.9, 148.5, 142.7, 139.8, 137.5,126.4, 124.9, 124.8, 120.9, 119.4, 118.4, 113.3, 109.0, 101.6, 85.7, 63.1, 55.5, 50.3, 40.1, 39.9, 39.7, 39.2, 39.0, 38.8ppm; MS (ES) m/z [M++l] 335.
Filed under: Uncategorized Tagged: azd 1080
