
CEP-18770, Delanzomib
cas 847499-27-8
Chemical Formula: C21H28BN3O5
Exact Mass: 413.21220, UNII-6IF28942WO;
CT-47098
NPH 007098
NPH007098
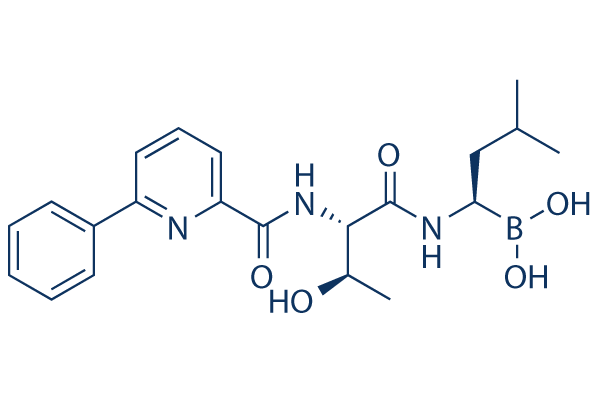
[(1R)-1-[[(2S,3R)-3-Hydroxy-2-[[(6-phenylpyridin-2-yl)carbonyl]amino]-1-oxobutyl]amino]-3-methylbutyl]boronic acid
[(lR)-l-[[(2S,3R)-3-hydroxy-2- [6-phenyl-pyridine-2-carbonyl)amino]-l-oxobutyl]amino]-3-methylbutylboronic acid,
Boronic acid, ((1R)-1-(((2S,3R)-3-hydroxy-1-oxo-2-(((6-phenyl-2-pyridinyl)carbonyl)amino)butyl)amino)-3-methylbutyl)-

CEP-18770 is a reversible P2 threonine boronic acid inhibitor of the chymotrypsin-like activity of the proteasome. Displays anti-multimyeloma (MM) effect.
HPLC………http://www.apexbt.com/downloader/document/A4009/HPLC.pdf
NMR………http://www.apexbt.com/downloader/document/A4009/NMR.pdf
CLICK ON IMAGE FOR CLEAR VIEW
Delanzomib, also known as CEP-18770, is An orally bioavailable synthetic P2 threonine boronic acid inhibitor of the chymotrypsin-like activity of the proteasome, with potential antineoplastic activity. Proteasome inhibitor CEP 18770 represses the proteasomal degradation of a variety of proteins, including inhibitory kappaBalpha (IkappaBalpha), resulting in the cytoplasmic sequestration of the transcription factor NF-kappaB; inhibition of NF-kappaB nuclear translocation and transcriptional up-regulation of a variety of cell growth-promoting factors; and apoptotic cell death in susceptible tumor cell populations. In vitro studies indicate that this agent exhibits a favorable cytotoxicity profile toward normal human epithelial cells, bone marrow progenitors, and bone marrow-derived stromal cells relative to the proteasome inhibitor bortezomib. The intracellular protein IkappaBalpha functions as a primary inhibitor of the proinflammatory transcription factor NF-kappaB
New series of dipeptidyl boronate inhibitors of 20S proteasome were identified to be highly potent drug-like candidates with IC50 values of 1.2 and 1.6 nM, respectively, which showed better activities than the drug bortezomib on the market
ref
The potent, selective, and orally bioavailable threonine-derived 20S human proteasome inhibitor that has been advanced to preclinical development, [(1R)-1-[ [ (2S,3R)- 3-hydroxy-2-[ (6-phenylpyridine- 2-carbonyl) amino]-1 -oxobutyl] amino]- 3-methylbutyl] boronic acid (CEP-18770, has been reported
ref .
Dorsey BD, Iqbal M, Chatterjee S, Menta E, Bernardini R, Bernareggi A, et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J Med Chem. 2008;51:1068–1072. [PubMed]
Further, the anti-multiple myeloma protea-some inhibitor CEP-18770 enhanced the anti-myeloma activity of bortezomib and melphalan. The combination of anti-multiple myeloma proteasome inhibitor CEP-18770 intravenously and bortezomib exhibited complete regression of bortezomib-sensitive tumours. Moreover, this combination markedly delayed progression of bortezomib-resistant tumours compared to treatment with either agent alone
PAPER
Discovery of a Potent, Selective, and Orally Active Proteasome Inhibitor for the Treatment of Cancer
http://pubs.acs.org/doi/abs/10.1021/jm7010589

The ubiquitin−proteasome pathway plays a central role in regulation of the production and destruction of cellular proteins. These pathways mediate proliferation and cell survival, particularly in malignant cells. The successful development of the 20S human proteasome inhibitor bortezomib for the treatment of relapsed and refractory multiple myeloma has established this targeted intervention as an effective therapeutic strategy. Herein, the potent, selective, and orally bioavailable threonine-derived 20S human proteasome inhibitor that has been advanced to preclinical development, [(1R)-1-[[(2S,3R)-3-hydroxy-2-[(6-phenylpyridine-2-carbonyl)amino]-1-oxobutyl]amino]-3-methylbutyl]boronic acid 20 (CEP-18770), is disclosed.
Patent
http://www.google.com/patents/WO2010056733A1?cl=en
Preferred among these compounds is [(lR)-l-[[(2S,3R)-3-hydroxy-2- [6-phenyl-pyridine-2-carbonyl)amino]-l-oxobutyl]amino]-3-methylbutylboronic acid, also known as CEP- 18770, which has the following structure:
PATENT
http://www.google.co.in/patents/WO2005021558A2
NOT SAME BUT SIMILAR
Example E.4 Boronic acid, [(lR)-l-[[(2S,3R)-3-hydroxy-2-[[4-(3-pyridyl)benzoyl]amino]-l- oxobutyI]amino]-3-methyIbutyl].
[00275] A mixture of 4-(pyridin-3-yl)benzamide, N-[(1S,2R)-1-[[[(1R)-1-
[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methano-l,3,2-benzodioxaborol-2- yl]-3-methylbutyl]amino]carbonyl]-2-hydroxypropyl]- of Example D.8.3 (155 mg, 0.283 mmol), 2-methylpropylboronic acid (81 mg, 0.793 mmol) and 2N aqueous hydrochloric acid (0.3 ml) in a heterogeneous mixture of methanol (3 ml) and hexane (3 ml) was stirred at room temperature for 24 hours. The hexane layer was removed and the methanolic layer was washed with fresh hexane (about 5 ml). Ethyl acetate (10 ml) was added to the methanol layer which was then concentrated. The residue was taken up with ethyl acetate and the mixture was concentrated. This step was repeated (2-3 times) until an amorphous white solid was obtained. The solid was then triturated with diethyl ether (5 ml) and the surnatant was removed by decantation. This step was repeated. The residue (126 mg) was combined with the product of a similar preparation (140 mg) and dissolved in ethyl acetate (about 40 ml) and a small amount of methanol (2-3 ml). The solution was washed with a mixture of NaCl saturated solution (7 ml) and 10% NaHCO3 (2 ml). The layers were separated and the aqueous phase was further washed with ethyl acetate (2 x 20 ml). The combined organic phases were dried over sodium sulfate and concentrated. The residue was taken up with ethyl acetate (about 20 ml) and the minimum amount of methanol, and then concentrated to small volume (about 5 ml). The resulting white was collected by filtration and dried under vacuum at 50°C (160 mg, 65% overall yield).
1H NMR (MeOH-d4): 8.90 (IH, s); 8.49 (IH, d, J=4.0); 8.20 (IH, d, J=8.1); 8.06 (2H, d, J=8.1); 7.85 (2H, d, J=8.1); 7.58 (IH, t br., J=6.0); 4.80 (IH, d, J=3.9); 4.40-4.29 (IH, m); 2.78 (IH, t, J=7.5); 1.73-1.61 (IH, m); 1.38 (2H, t, J=6.9); 1.31 (3H, d, J=6.3); 0.94 (6H, d, J=6.31). [00276] Further compounds prepared according to the above procedure for
Example E.4 are reported in Table E-4. Table E-4
E.4.3 IS THE COMPD
|
References |
1. Fuchs, Ota. Proteasome inhibition as a therapeutic strategy in patients with multiple myeloma. Multiple Myeloma (2009), 101-125. CODEN: 69MVM2 AN 2010:737549
2. Genin, E.; Reboud-Ravaux, M.; Vidal, J. Proteasome inhibitors: recent advances and new perspectives in medicinal chemistry. Current Topics in Medicinal Chemistry (Sharjah, United Arab Emirates) (2010), 10(3), 232-256. CODEN: CTMCCL ISSN:1568-0266. CAN 152:516315 AN 2010:423458
3. Sanchez, Eric; Li, Mingjie; Steinberg, Jeffrey A.; Wang, Cathy; Shen, Jing; Bonavida, Benjamin; Li, Zhi-Wei; Chen, Haiming; Berenson, James R. The proteasome inhibitor CEP-18770 enhances the anti-myeloma activity of bortezomib and melphalan. British Journal of Haematology (2010), 148(4), 569-581. CODEN: BJHEAL ISSN:0007-1048. AN 2010:353952
4. Dick, Lawrence R.; Fleming, Paul E. \Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discovery Today (2010), 15(5/6), 243-249. CODEN: DDTOFS ISSN:1359-6446. AN 2010:318415
5. Ruggeri, Bruce; Miknyoczki, Sheila; Dorsey, Bruce; Hui, Ai-Min. The development and pharmacology of proteasome inhibitors for the management and treatment of cancer. Advances in Pharmacology (San Diego, CA, United States) (2009), 57(Contemporary Aspects of Biomedical Research: Drug Discovery), 91-135. CODEN: ADPHEL ISSN:1054-3589. AN 2010:62762
6. Chen-Kiang, Selina; Di Liberto, Maurizio; Huang, Xiangao. Targeting CDK4 and CDK6 kinases or genes thereof in cancer therapy for sensitizing drug-resistant tumors. PCT Int. Appl. (2009), 149pp. CODEN: PIXXD2 WO 2009061345 A2 20090514 CAN 150:531264 AN 2009:586623
7. Rickles, Richard; Lee, Margaret S. Use of adenosine A2A receptor agonists and phosphodiesterase (PDE) inhibitors for the treatment of B-cell proliferative disorders, and combinations with other agents. PCT Int. Appl. (2009), 70 pp. CODEN: PIXXD2 WO 2009011893 A2 20090122 CAN 150:160095 AN 2009:86451
8. Rickles, Richard; Pierce, Laura; Lee, Margaret S. Combinations for the treatment of B-cell proliferative disorders. PCT Int. Appl. (2009), 79pp. CODEN: PIXXD2 WO 2009011897 A1 20090122 CAN 150:160094 AN 2009:83374
9. Hoveyda, Hamid; Fraser, Graeme L.; Benakli, Kamel; Beauchemin, Sophie; Brassard, Martin; Drutz, David; Marsault, Eric; Ouellet, Luc; Peterson, Mark L.; Wang, Zhigang. Preparation and methods of using macrocyclic modulators of the ghrelin receptor. U.S. Pat. Appl. Publ. (2008), 178pp. CODEN: USXXCO US 2008194672 A1 20080814 CAN 149:288945 AN 2008:975261
10. Piva, Roberto; Ruggeri, Bruce; Williams, Michael; Costa, Giulia; Tamagno, Ilaria; Ferrero, Dario; Giai, Valentina; Coscia, Marta; Peola, Silvia; Massaia, Massimo; Pezzoni, Gabriella; Allievi, Cecilia; Pescalli, Nicoletta; Cassin, Mara; di Giovine, Stefano; Nicoli, Paola; de Feudis, Paola; Strepponi, Ivan; Roato, Ilaria; Ferracini, Riccardo; Bussolati, Benedetta; Camussi, Giovanni; Jones-Bolin, Susan; Hunter, Kathryn; Zhao, Hugh; Neri, Antonino; Palumbo, Antonio; Berkers, Celia; Ovaa, Huib; Bernareggi, Alberto; Inghirami, Giorgio. CEP-18770: a novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood (2008), 111(5), 2765-2775. CODEN: BLOOAW ISSN:0006-4971. CAN 149:486154 AN 2008:292777
11. Dorsey, Bruce D.; Iqbal, Mohamed; Chatterjee, Sankar; Menta, Ernesto; Bernardini, Raffaella; Bernareggi, Alberto; Cassara, Paolo G.; D’Arasmo, Germano; Ferretti, Edmondo; De Munari, Sergio; Oliva, Ambrogio; Pezzoni, Gabriella; Allievi, Cecilia; Strepponi, Ivan; Ruggeri, Bruce; Ator, Mark A.; Williams, Michael; Mallamo, John P. Discovery of a Potent, Selective, and Orally Active Proteasome Inhibitor for the Treatment of Cancer. Journal of Medicinal Chemistry (2008), 51(4), 1068-1072. CODEN: JMCMAR ISSN:0022-2623. CAN 148:345774 AN 2008:146611
12. Dorsey, Bruce D.; Menta, Ernesto; Bernardini, Raffaella; Bernareggi, Alberto; Casara, Paolo G.; D’Arasmo, Germano; Ferretti, Edmondo; De Munari, Sergi; Oliva, Ambrogio; Iqbal, Mohamed; Chatterjee, Sankar; Ruggeri, Bruce; Ator, Mark A.; Williams, Michael; Mallamo, John P. CEP-18770: Discovery of a Potent, Selective and Orally Active Proteasome Inhibitor for the Treatment of Cancer. Frontiers in CNS and Oncology Medicinal Chemistry, ACS-EFMC, Siena, Italy, October 7-9 (2007), COMC-027. CODEN: 69KAR2 AN 2007:1171000
13. Marblestone Jeffrey G Ubiquitin Drug Discovery & Diagnostics 2009 – First Annual Conference. IDrugs : the investigational drugs journal (2009), 12(12), 750-3.
| Patent | Submitted | Granted |
|---|---|---|
| Proteasome inhibitors and methods of using the same [US7576206] | 2005-05-19 | 2009-08-18 |
| PROTEASOME INHIBITORS AND METHODS OF USING THE SAME [US7915236] | 2009-11-26 | 2011-03-29 |
| BORONATE ESTER COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF [US2009325903] | 2009-12-31 |
| US7442830 * | 6 Aug 2007 | 28 Oct 2008 | Millenium Pharmaceuticals, Inc. | Proteasome inhibitors |
| US7687662 * | 2 Jul 2008 | 30 Mar 2010 | Millennium Pharmaceuticals, Inc. | Proteasome inhibitors |
| US8003819 * | 12 Feb 2010 | 23 Aug 2011 | Millennium Pharmaceuticals, Inc. | Proteasome inhibitors |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8962572 | 4 Oct 2011 | 24 Feb 2015 | Fresenius Kabi Usa, Llc | Bortezomib formulations |
| WO2012177835A1 | 21 Jun 2012 | 27 Dec 2012 | Cephalon, Inc. | Proteasome inhibitors and processes for their preparation, purification and use |
/////CEP-18770, delanzomib
B(C(CC(C)C)NC(=O)C(C(C)O)NC(=O)C1=CC=CC(=N1)C2=CC=CC=C2)(O)O
Filed under: Uncategorized Tagged: CEP-18770, CT 47098, Delanzomib, NPH 007098, NPH007098




