|
|||
|
|||
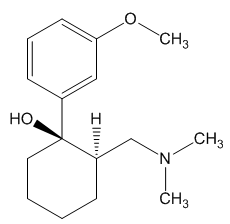
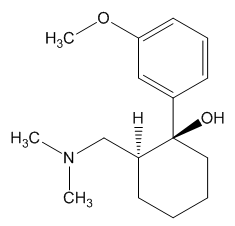
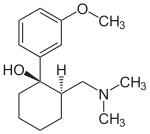
2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol
Tramadol (marketed as Ultram, and as generics) is an opioid pain medication used to treat moderate to moderately severepain.[1] When taken as an immediate-release oral formulation, the onset of pain relief usually occurs within about an hour.[5]It has two different mechanisms. First, it binds to the μ-opioid receptor. Second, it inhibits the reuptake of serotonin andnorepinephrine.[6][7]
Serious side effects may include seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction. Common side effects include: constipation, itchiness and nausea, among others. A change in dosage may be recommended in those with kidney or liver problems. Its use is not recommended in women who are breastfeeding or those who are at risk of suicide.[1]
Tramadol is marketed as a racemic mixture of both R– and S–stereoisomers.[2] This is because the two isomers complement each other’s analgesic activity.[2] It is often combined with paracetamol (acetaminophen) as this is known to improve the efficacy of tramadol in relieving pain.[2] Tramadol is metabolised to O-desmethyltramadol, which is a more potent opioid.[8] It is of the benzenoid class.
Tramadol was launched and marketed as Tramal by the German pharmaceutical company Grünenthal GmbH in 1977 inWest Germany, and 20 years later it was launched in countries such as the UK, U.S., and Australia.[7]
Developed (from 1962) by the German company Grünenthal, and is marketed through much of the world under various trade names, including Acugersic (Malaysia), Mabron (some Eastern European countries as well as parts of the Middle and Far East), Ultram (USA), Zaldiar (France and much of Europe, as well as Russia) and Zydol (UK and Ireland).
CONFUSION ON CIS TRANS
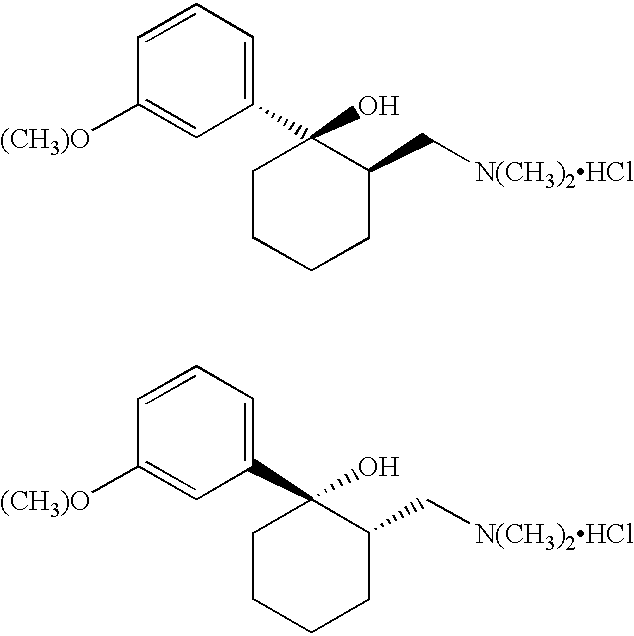
There is some confusion within the literature as to what should be called cis and what should be called trans. For purposes of this disclosure, what is referred to herein as the trans form of Tramadol includes the R,R and S,S isomers as shown by the following two structures:
The cis form of Tramadol, as that phrase is used herein, includes the S,R and the R,S isomers which are shown by the following two structures:
Tramadol is marketed as a racemic mixture of both R and S stereoisomers. It is a μ-opioid receptor agonist, like morphine, but much less active. It inhibits reuptake of the neurotransmitters serotonin and norepinephrine, suggesting that it lifts mood and thereby may dull the brain’s perception of pain.
 |
 |
|
1R,2R-Tramadol |
1S,2S-Tramadol |
In the body, tramadol undergoes demethylation to several metabolites by a Cytochrome P450 enzyme (CYP2D6) in the liver, the most important of these products being O-desmethyltramadol. O-desmethyltramadol has a much stronger (200x) affinity for the μ-opioid receptor than tramadol, so in effect tramadol is a prodrug.
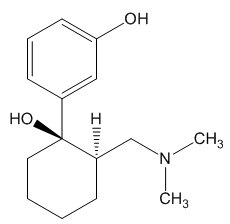 |
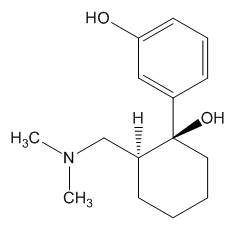 |
|
1R,2R–O-desmethyltramadol |
1S,2S–O-desmethyltramadol |
Not everyone’s liver works identically. Around 6% of the Caucasian population has a reduced CYP2D6 activity (hence reducing metabolism), so there is a reduced analgesic effect with Tramadol. These people require a dose increase of 30% to get the same pain relief as the norm. A case has been reported of a patient where, following an overdose, their ultrarapid tramadol metabolism led to excessive norepinephrine levels, with near-fatal consequences.
However, it has recently been discovered at relatively high concentrations in the roots of the African peach or pin cushion tree (Nauclea latifolia), which has a long tradition as a folk remedy. As usual, Nature got there first.

The African pin-cushion tree (Nauclea latifolia)
In the area of “legal highs”, a disturbing development is a drug blend known as “Krypton”. This isn’t the noble gas, but a mixture of O-desmethyltramadol withKratom (Mitragyna speciosa, a medicinal plant that originates in SE Asia, seemingly the local equivalent of khat), which contains an alkaloid mitragynine which is also a μ-receptor agonist. Several fatalities have been linked with its use, notably in Sweden.
SYNTHESIS

(1R,2R)-Tramadol (1S,2S)-Tramadol


(1R,2S)-Tramadol (1S,2R)-Tramadol
The chemical synthesis of tramadol is described in the literature.[35] Tramadol [2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol] has two stereogenic centers at thecyclohexane ring. Thus, 2-(dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexanol may exist in four different configurational forms:
- (1R,2R)-isomer
- (1S,2S)-isomer
- (1R,2S)-isomer
- (1S,2R)-isomer
The synthetic pathway leads to the racemate (1:1 mixture) of (1R,2R)-isomer and the (1S,2S)-isomer as the main products. Minor amounts of the racemic mixture of the (1R,2S)-isomer and the (1S,2R)-isomer are formed as well. The isolation of the (1R,2R)-isomer and the (1S,2S)-isomer from the diastereomeric minor racemate [(1R,2S)-isomer and (1S,2R)-isomer] is realized by the recrystallization of the hydrochlorides. The drug tramadol is a racemate of the hydrochlorides of the (1R,2R)-(+)- and the (1S,2S)-(–)-enantiomers. The resolution of the racemate [(1R,2R)-(+)-isomer / (1S,2S)-(–)-isomer] was described[36] employing (R)-(–)- or (S)-(+)-mandelic acid. This process does not find industrial application, since tramadol is used as a racemate, despite known different physiological effects[37] of the (1R,2R)- and (1S,2S)-isomers, because the racemate showed higher analgesic activity than either enantiomer in animals[38] and in humans.[39]
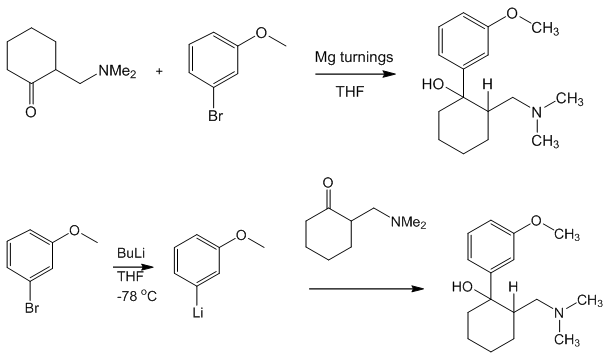
Synthesised by chemists at the German company Grünenthal and brought to the market in 1977. It can readily be made by nucleophilic attack of a Grignard or RLi species upon a carbonyl group.
ALSO

Paper
http://www.jmcs.org.mx/PDFS/V49/N4/04-Alvarado.pdf
Tramadol hydrochloride (1). To a solution of 3-bromoanisol 13 (0.823 g, 4.4 mmol) in dry THF (10 mL), 1.75 M n-BuLi (2.5 mL, 4.4 mmol) was added dropwise at -78°C under argon atmosphere. The mixture was stirred at the same temperature during 45 minutes and a solution of 2-dimethylaminomethylcyclohexanone 6a (0.62 g, 4mmol) in dry THF was added dropwise. The resulting mixture was stirred at -78°C for 2 h. and the solvent was removed in vacuo. Water (30 mL) was added and the product was extracted with ethyl ether (3X30 mL). The extracts were dried over sodium sulfate, filtered and evaporated in vacuum. The residue was treated with 5mL of ethyl ether saturated with hydrogen chloride; the ethyl ether was evaporated in vacuo and the resulting solid was purified by crystallization from acetone. Tramadol hydrochloride 1 was obtained as white crystals (0.94 g, 78.6%),
MP 168- 175°C.
IR (KBr): 3410, 3185, 2935, 2826, 2782, 1601, 1249, 702 cm-1;
1H NMR (CDCl3, 300 MHz) δ 1.2-1.9 (10H, m), 2.15 (6H, s), 2.45 (1H, dd, J = 15.1, 4.4 Hz), 3.82 (3H, s), 6.76 (1H, dd, J = 8, 2.4 Hz), 7.04 (1H, d, 7.6 Hz), 7.14 (1H, s), 7.26 (1H, t, 3.9 Hz), 11.4 (1H, bs);
MS (EI): m/z 263 M+ (28), 58 (100).
PATENT
http://www.google.com.ar/patents/WO1999003820A1?cl=en
Tramadol is the compound cis(+/-)-2-[(dimethylamino)-methyl]-l-(3- methoxyphenyl) cyclohexanol which, in the form of the hydrochloride salt is widely used as an analgesic
Tramadol means the racemic mixture of cis-Tramadol as shown by the following chemical structures:
(1 R, 2R) (1S. 2S) cis-Tramadol
Step l Formation of Dimethylaminomethyl Cyclohexanone Hydrochloride
Dimethylaminomethyl Cyclohexanone Hydrochloride
Step 2 Formation of Tramadol Mannich Base
NaOH, Water
Toluene, TBME
Tramadol Mannich Base
Step 3 Formation of Tramadol Base Hydrate
Tramadol Base Hydrate (crude) Step 4 Purification of Tramadol Base Hydrate
Tramadol Base Hydrate (pure)
Step 5 Formation of Tramadol Hydrochloride
Tramadol Hydrochloride
Example 1
To produce the Tramadol base hydrate, a reaction vessel is charged successively with 69 Kg of Magnesium, 400 1 of dry Tetrahydrofuran (THF) and 15 1 of 3- bromoanisole.
With careful heating, the reactor temperature is brought up to ca. 30°C. The Grignard initiates at this point and exotherms to approximately 50°C. A further 5 1 of bromoanisole are added which maintains reflux. 400 1 of THF are then added before the remainder of the bromoanisole. This addition of the remainder of the bromoanisole is carried out slowly so as to sustain a gentle reflux. The reaction is refluxed after complete addition of 3-bromoanisole. The vessel is cooled and Mannich base is added. When addition is complete, the vessel is reheated to reflux for 30 minutes to ensure complete reaction. After cooling to ca. 10°C, 2,300 1 of water are added to quench the reaction. When complete, part of the solvents are distilled under vacuum. Approximately 260 1 of concentrated HC1 is added at a low temperature until a pH of 0 – 1 is reached. This aqueous phase is extracted with toluene. The toluene phases are discarded and ethyl acetate is added to the aqueous phase. 30% Ammonia solution is then charged to reach pH 9 – 10 and the phases are separated. The aqueous phases are extracted again with ethyl acetate and finally all ethyl acetate layers are combined and washed twice with water. Ethyl acetate is then distilled from the reaction solution at atmospheric pressure. Process water is added and the solution cooled to 20°C and seeded. After crystallisation, the vessel is cooled to -5 to 0°C and stirred for one hour.
The product is centrifuged at this temperature and washed with cold ethyl acetate 5 x 50 1. Approximately 310 – 360 Kg of moist cis-Tramadol base hydrate are obtained.
Purification
A reactor vessel is charged successively with cis-Tramadol base hydrate (crude) 200 Kg and ethyl acetate 300 1 and the contents of the vessel heated to 50°C until all solids are in solution. The vessel is then cooled to -5 to 0°C and the product crystallises. Stirring is continued for two hours and the product is then centrifuged and washed with cold ethyl acetate, 2 x 25 1. Approximately 165 – 175 Kg (moist) of cis(+/-) Tramadol base hydrate are obtained from this procedure.
The overall process produced high yields of cis-Tramadol with a trans isomer content of less than 0.03%. Analytical data of the base hydrate of cis-Tramadol
Melting point: 79 – 80°C (in comparison cis-Tramadol base anhydrous is an oil). Water content (KF) : 6.52% (= monohydrate) IR-spectrum of the base hydrate of cis-Tramadol (see Fig. 1).
IR-spectrum (=cis-Tramadol base anhydrous, see Fig. 2).
The invention provides a unique process in which a base hydrate of cis-Tramadol is selectively crystallised without impurities. The base hydrate is processed to readily form cis-Tramadol hydrochloride. The process is substantially simpler than known processes and does not require the use of potentially toxic solvents. Thus the process is environmentally friendly.
The base hydrate of cis-Tramadol prepared may also be used in various formulations.
The base hydrate of cis-Tramadol may be formulated in the form of a solid with a slow release profile. For example, slow release pellets may be prepared by coating a suitable core material with a coating, for example, of ethylcellulose/schellack solution (4:1) and suitable pharmaceutical excipients. The pellets have typical average diameter of 0.6 to 1.6 mm. The pellets may be readily converted into gelatine capsules or pressed into tablet form using well-known techniques.
Alternatively the base hydrate of cis-Tramadol may be formulated into effervescent tablets by forming granules of the base hydrate with acidity/taste modifiers and a suitable effervescent base such as sodium hydrogen carbonate /anhydrous sodium carbonate (12:1). The ingredients are typically blended in a mixer /granulator and heated until granulation occurs. The resulting granules may be pressed into tablet form, on cooling. Of particular interest is the use of the base hydrate of cis-Tramadol in a form for parenteral use/injectables. The base hydrate is typically dissolved in water together with suitable excipients (as necessary). The solution is filtered through a membrane to remove solid fibres or particles. The filtered solution may then be filled into ampoules, typically containing 10.0 mg of the active compound. Usually the formulation is prepared for intramuscular injection.
PATENT
http://www.google.com/patents/EP1346978A1?cl=en
-
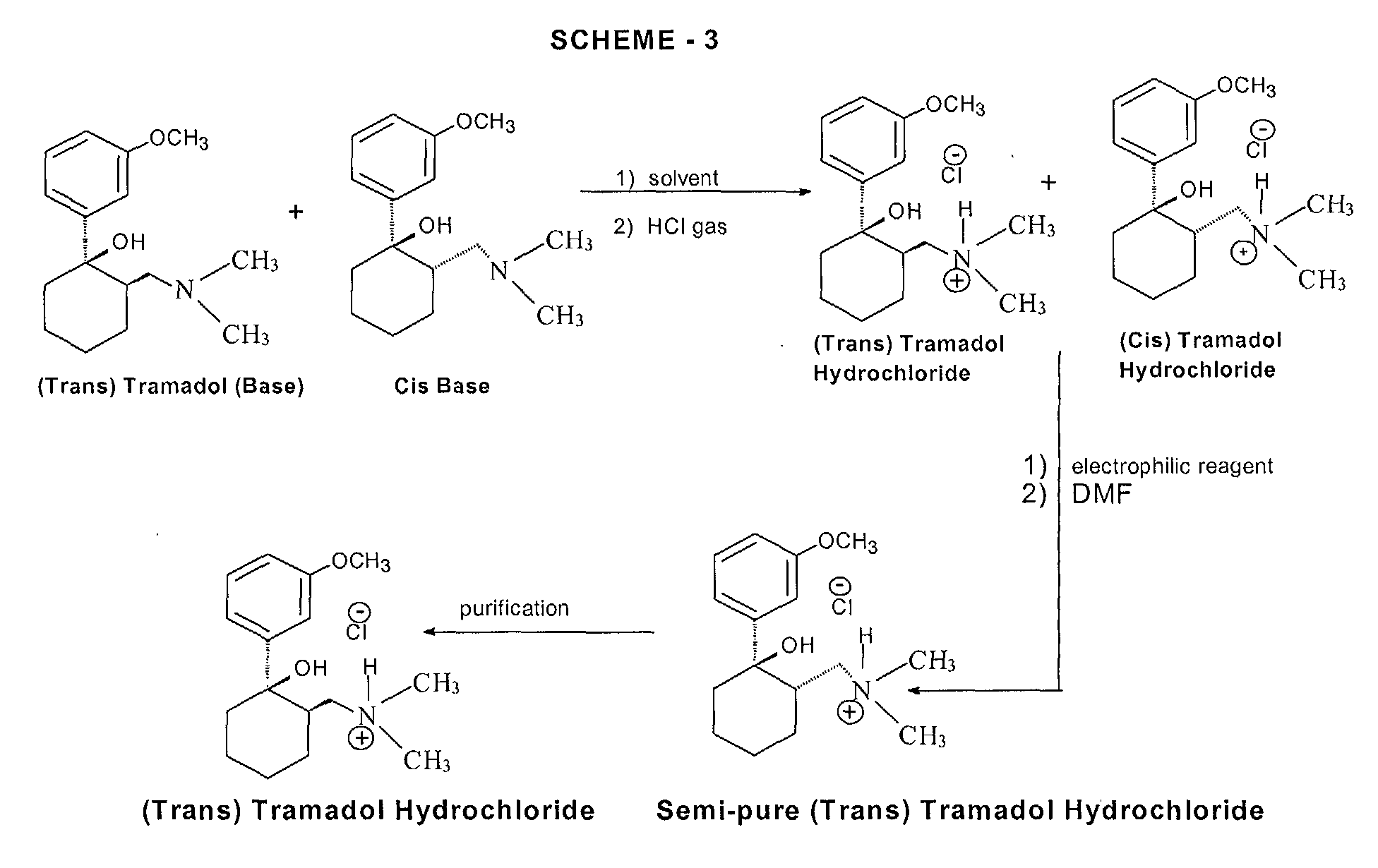
Various processes for the synthesis of tramadol hydrochloride have been described in the prior art. For example, US 3 652 589 and British patent specification no. 992 399 describe the preparation of tramadol hydrochloride. In this method, Grignard reaction of 2-dimethylaminomethyl cyclohexanone (Mannich base) with metabromo-anisole gives an oily mixture of tramadol and the corresponding cis isomer, along with Grignard impurities. This oily reaction mixture is subjected to high vacuum distillation at high temperature to give both the geometric isomers of the product base as an oil. This oil, on acidification with hydrogen chloride gas, furnishes insufficiently pure tramadol hydrochloride as a solid. This must then be purified, by using a halogenated solvent and 1,4-dioxane, to give sufficiently pure tramadol hydrochloride. The main drawback of this process is the use of large quantities of 1,4-dioxane and the need for multiple crystallizations to get sufficiently pure trans isomer hydrochloride (Scheme – 1).
-
[0004]
The use of dioxane for the separation of tramadol hydrochloride from the corresponding cis isomer has many disadvantages, such as safety hazards by potentially forming explosive peroxides, and it is also a category 1 carcinogen (Kirk and Othmer, 3rd edition, 17, 48). Toxicological studies of dioxane show side effects such as CNS depression, and necrosis of the liver and kidneys. Furthermore, the content of dioxane in the final tramadol hydrochloride has been strictly limited; for example, the German Drug Codex (Deutscher Arzneimittel Codex, DAC (1991)) restricts the level of dioxane in tramadol hydrochloride to 0.5 parts per million (ppm).
-
In another process, disclosed in US patent specification no. 5 414 129, the purification and separation of tramadol hydrochloride is undertaken from a reaction mixture containing the trans and cis isomers, and Grignard reaction side products, in which the reaction mixture is diluted in isopropyl alcohol and acidified with gaseous hydrogen chloride to yield (trans) tramadol hydrochloride (97.8%) and its cis isomer (2.2%), which is itself crystallized twice with isopropyl alcohol to give pure (trans) tramadol hydrochloride (Scheme – 2). This process relies on the use of multiple solvents to separate the isomers (ie butylacetate, 1-butanol, 1-pentanol, primary amyl alcohol mixture, 1-hexanol, cyclohexanol, 1-octanol, 2-ethylhexanol and anisole). The main drawback of this process is therefore in using high boiling solvents; furthermore, the yields of tramadol hydrochloride are still relatively low and the yield of the corresponding cis hydrochloride is relatively high in most cases.
-
PCT patent specification no. WO 99/03820 describes a method of preparation of tramadol (base) monohydrate, which involves the reaction of Mannich base with metabromo-anisole (Grignard reaction) to furnish a mixture of tramadol base with its corresponding cis isomer and Grignard impurities. This, on treatment with an equimolar quantity of water and cooling to 0 to -5°C, gives a mixture of tramadol (base) monohydrate with the corresponding cis isomer (crude). It is further purified with ethyl acetate to furnish pure (trans) tramadol (base) monohydrate, which is again treated with hydrochloric acid in the presence of a suitable solvent to give its hydrochloride salt (Scheme – 2). The drawback of this method is that, to get pure (trans) tramadol hydrochloride, first is prepared pure (trans) tramadol (base) monohydrate, involving a two-step process, and this is then converted to its hydrochloride salt. The overall yield is low because of the multiple steps and tedious process involved.
-
More recently, a process for the separation of tramadol hydrochloride from a mixture with its cis isomer, using an electrophilic reagent, has been described in US patent specification no. 5 874 620. The mixture of tramadol hydrochloride with the corresponding cis isomer is reacted with an electrophilic reagent, such as acetic anhydride, thionyl chloride or sodium azide, using an appropriate solvent (dimethylformamide or chlorobenzene) to furnish a mixture of tramadol hydrochloride (93.3 to 98.6%) with the corresponding cis isomer (1.4 to 6.66%), (Scheme – 3). The product thus obtained is further purified in isopropyl alcohol to give pure (trans) tramadol hydrochloride. However, the drawback of this process is that a mixture of tramadol base with its cis isomer is first converted into the hydrochloride salts, and this is further reacted with toxic, hazardous and expensive electrophilic reagents to get semi-pure (trans) tramadol hydrochloride. The content of the cis isomer is sufficiently high to require further purification, and this therefore results in a lower overall yield.
-
Therefore, all the known methods require potentially toxic solvents and/or reagents, and multiple steps to produce the desired quality and quantity of tramadol hydrochloride. By contrast, the present invention requires a single step process (or only two steps when tramadol hydrochloride is made via the tramadol (base) monohydrate route) using a natural solvent (ie water) in the absence of carcinogenic solvents (such as the category 1 carcinogen, 1,4-dioxane) to produce pure tramadol hydrochloride, so it is ‘ecofriendly’ and easily commercialized to plant scale without any difficulties.
PATENT
http://www.google.co.in/patents/US6399829

EXAMPLE 8 Hydrochloride Formed without Improvement of the Trans:Cis Ratio
Whether a recrystallization step improves the trans:cis ratio of Tramadol depends upon the solvent composition from which the recrystallization is performed. When the hydrochloride form of Tramadol is produced and then crystallized in the presence of a solvent with a high toluene concentration, the ratio of trans:cis remains essentially unchanged. This is in contrast to the recrystallization from a solvent which has a high acetonitrile concentration as was the case in Examples 5-7.
A 21 mL solution of 1.8 g of HCl gas (bubbled at 5° C.) in acetonitrile (yielding a 2.0 M solution), was added to 10.2 g of Grignard product C (90/10 of trans/cis) in 30 mL of toluene and stirred mechanically for 3 hours. The mixture was filtered and washed with toluene. Drying in vacuo yielded 11.2 g (96% recovery). The resulting hydrochloride had a trans/cis ratio of 92:8, essentially the same trans:cis ratio as did the 10.2 g of Grignard product C.
Recrystallization from 90 mL of acetonitrile yielded 8.83 g, which was 96.6/3.4 of trans/cis by HPLC. Of this, 8.6 g was recrystallized from 75 mL of acetonitrile to give 7.44 g, trans/cis ratio of 99.6/0.4.
This example shows that the formation of the hydrochloride in the presence of a relatively large amount of toluene (here about 60%) and crystallization from toluene-acetonitrile does not improve the trans:cis ratio. As the percentage of toluene present in the mixture of toluene and acetonitrile in a crystallization step is decreased, the trans:cis ratio of the recovered product will increase. Steps in which the hydrochloride is recrystallized from acetonitrile do yield an improved trans:cis ratio.
PATENT
https://www.google.com/patents/EP0831082A1
The synthesis of Tramadol is described in U.S. Patent No. 3,652,589 and in British Patent No. 992,399. The synthesis of Tramadol consists of a Grignard reaction between 2-dimethylaminomethylcyclohexanone and 3-methoxyphenyl magnesium bromide (Equation 1). From the reaction scheme, it is clear that both isomers (RR,SS) (Structure 1) and (RS, SR) (Structure 2) are obtained in variable ratios, depending on the reaction conditions.
The original patents assigned to Gruenenthal GmbH describe the isolation of the (RR,SS) isomer, as follows:
The complex mixture of products containing both isomers of Tramadol obtained from the Grignard reaction is distilled under reduced pressure. The isomers are distilled together at 138-140°C (0.6 mm Hg). The distillate is dissolved in ether and is reacted with gaseous HCl. The resulting mixture of both isomers of Tramadol is precipitated as hydrochlorides and filtered. The resulting mixture contains about 20% of the (RS,SR) isomer. The isomer mixture is then refluxed twice with five volumes of moist dioxane, and filtered. The cake obtained consists of pure (RR,SS) isomer. The residual solution consists of “a mixture of about 20-30% of the cis (i.e. RS,SR), which cannot be further separated by boiling dioxane” [U.S. Patent 3,652,589, Example 2].
Dioxane, used in large quantities in this process, possesses many undesirable properties. It has recently been listed as a Category I carcinogen by OSHA [Kirk & Othmer, 3rd Ed., Vol. 9, p. 386], and it is known to cause CNS depression and liver necrosis [ibid., Vol. 13, p. 267]; in addition, it tends to form hazardous peroxides [ibid., Vol 17, p. 48]. As a result, the concentration of dioxane in the final product has been strictly limited to several ppb’s, and the DAC (1991) restricted the level of dioxane in Tramadol to 0.5 ppm.
A different separation method, described in Israeli Specification No. 103096, takes advantage of the fact that the precipitation of the (RR,SS) isomer of Tramadol from its solution in medium chained alcohols (C4-C8) occurs faster than the precipitation of the (RS,SR) isomer, which tends to separate later. The main disadvantage of this method is, that the time interval between the end of separation of the (RR,SS) isomer and the beginning of the (RS,SR) isomer separation is variable, and seems to depend sharply on the composition of the crude mixture. Therefore, variations in the yield and the quality of the product often occur. Furthermore, about 40% of the (RR,SS) isomer does not separate and remains in solution, along with the (RS,SR) isomer. This remaining mixture cannot be further purified by this method.
Another method, described in Israeli Specification No. 116281, relies on the fact that the (RS,SR) isomer of Tramadol undergoes dehydration much faster then the (RR,SS) isomer, when treated with 4-toluenesulfonic acid, or sulfuric acid; furthermore, when the reaction is carried out in an aqueous medium, a certain amount (up to 50%) of the (RS,SR) isomer is converted to the (RR,SS) isomer. This may, of course increase the efficiency of the process.
The unreacted (RR, SS) isomer is then separated from the dehydrated products and from other impurities by simple crystallization.
While further examining the results of the latter process, it was surprisingly found that the hydroxyl group of the (RS,SR) isomer of Tramadol reacts faster than the same group of the (RR,SS) with various reagents. A plausible explanation for this observation can be supplied by comparing the structures of both isomers, and their ability to form hydrogen bonds.
Looking closely at Fig. 1 [(RR,SS) Tramadol hydrochloride] and at Fig. 2 [(RS,SR) Tramadol hydrochloride], one can provide a plausible explanation for the difference in the OH group’s activity, as follows: The proton attached to the nitrogen atom of the protonated (RR,SS) isomer of Tramadol is capable of forming a stable hydrogen bonding with the oxygen atom of the hydroxyl group (see Fig. 1). Thus, any reaction involving protonation of the hydroxyl group (such as dehydration), or any reaction in which the hydroxyl group reacts as a nucleophile (such as a nucleophilic substitution or esterification process) is less favored to occur.
In the (RS,SR) isomer, on the other hand, there is no possible way of forming a stable intramolecular hydrogen bond, and therefore, any of the above-mentioned types of reactions can easily occur, considering the fact that this particular hydroxyl group is tertiary and benzyllic.
The general purification procedure of the present invention consists of reacting a mixture of both geometrical isomers of Tramadol hydrochloride with a potential electrophile under such conditions that the (RS,SR) isomer reacts almost exclusively, while the (RR,SS) isomer remains practically intact. The resulting mixture is evaporated and the resulting solid substance is then recrystallized from isopropanol or any other suitable solvent.
Example 1
11.1 g of a mixture consisting of 77% (RR,SS) Tramadol hydrochloride and 23% of the corresponding (RS,SR) isomer were dissolved in 30 ml DMF. 1.3 g acetic anhydride were added and the reaction mixture was stirred at room temperature for 12 hours. The solvent was partly evaporated under reduced pressure and 15 ml toluene were added. The suspension obtained was filtered and washed with 5 ml toluene. 5.8 g of crystals were obtained, in which the (RR,SS):(RS,SR) isomer ratio was 70:1. The product obtained was crystallized from 12 ml isopropanol and 4 g of pure (RR,SS) Tramadol hydrochloride were obtained.
Example 2
19.5 g of a mixture consisting of 60.5% (RR,SS) Tramadol hydrochloride and 40.5% of the corresponding (RS,SR) isomer were suspended in 55 ml chlorobenzene. A solution of 4 ml thionyl chloride in 15 ml chlorobenzene was added dropwise for two hours. The suspension was partly evaporated, the residue was filtered and rinsed with toluene, and 8.1 g of crystals were obtained, in which the (RR,SS):(RS, SR) isomer ratio was 14:1. The product obtained was recrystallized from isopropanol, and 6.7 g of pure (RR,SS) Tramadol hydrochloride were obtained.
Example 3
33.4 g of a mixture consisting of 45% (RR,SS) Tramadol hydrochloride and 55% of the corresponding (RS,SR) isomer was immersed in 50 ml trifluoroacetic acid, 5.2 g of sodium azide was added, and the reaction mixture was stirred for 24 hours. The reaction mixture was then evaporated under reduced pressure, 50 ml water was added, and the solution was brought to pH 12 with solid potassium carbonate. The suspension was extracted with 50 ml toluene, the solvent was evaporated and 25 ml hydrogen chloride solution in isopropanol were added. The solution was cooled and filtered. 9.5 g of crude (RR,SS) Tramadol were obtained, and the crude product was purified by recrystallization from isopropanol.
The hitherto unknown (RS,SR)-2-(dimethylaminomethyl)-1-azido-1-(3-methoxyphenyl)-cyclohexane hydrochloride was isolated from the reaction mixture, recrystallized from isopropanol and characterized as follows:
(RS,SR)-2-(dimethylaminomethyl)-1-azido-1-(3-methoxyphenyl)-cyclohexane hydrochloride
- ms : 288 m+
- IR : 2050 cm-1 (N3)
- 1H-NMR (DMSO): 10.42 ppm: (acidic proton); 1H; 7.40-6.90 ppm: (aromatic protons) 4H; 3.79 ppm; (OCH3 ), 3H; 2.78, 2.42 ppm: NCH2 ; 2H; 2.58, 2.37 ppm: [N(CH3 )2], 6H; 2.30-1.40 ppm: cyclohexane ring protons, 9H.
- 13C-NMR (DMSO): 159.74 ppm: C1; 144.12 ppm: C5; 130.08 ppm: C3; 117.36 ppm: C4; 112.97, 111.35 ppm: C2, C6; 69.61 ppm: C8; 59.29 ppm: C14; 55.21 ppm: C7; 44.6 ppm: C15; 40.12 ppm: C13; 35.94, 27.02, 23.56, 21.63 ppm: cyclohexane ring carbon nucleii.
AZIDE COMPD
Bibliography
Synthesis of Tramadol
http://www.nioch.nsc.ru/icnpas98/pdf/posters1/156.pdf
- http://www.opioids.com/tramadol/synthesis/index.html
- Patents to Grünenthal G.m.b.H.:- K. Flick and E. Frankus, U.S. Patent 3,652,589, March 28, 1972; Chem. Abs., 1972, 76, 153321; K. Flick and E. Frankus, U.S. Patent. 3,830,934, August 20, 1974; Chem. Abs., (1974), 82, 21817 (synth)
- C. Alvarado, Á. Guzmán, E. Díaz and R. Patiño, J. Mex. Chem. Soc., 49, (2005) 324-327 (synth)
- G. Venkanna, G. Madhusudhan, K. Mukkanti, A. Sankar, V. M. Kumar and S. A. Ali, J. Chem. Pharm. Res., 4 (2012) 4506-4513 (synth)
- A. Boumendjel, G. S.Taïwe, E. N. Bum, T. Chabrol, C. Beney, V. Sinniger, R. Haudecoeur, L. Marcourt, S. Challal, E. F. Queiroz, F. Souard, M. Le Borgne, T. Lomberget, A. Depaulis, C. Lavaud, R. Robins, J.-L. Wolfender, B. Bonaz and M. De Waard, Angew. Chem. Int. Ed., 52 (2013) 11780 –11784 (Tramadol in nature)
Action
- R. B. Raffa, E. Friderichs, W. Reimann, R. P. Shank, E. E. Codd and J. L. Vaught, J. Pharmacol. Exp. Ther., 260 (1992) 275-285 (means of action)
- P. Dayer, L. Collart and J. Desmeules, Drugs (Suppl. 1), (1994) 3-7.
- T. A. Bamigbade, C. Davidson, R. M. Langford and J. A. Stamford, Br. J. Anaesthes., 79 (1997) 352-356. (action of enantiomers)
- P. W. Keeley, G. Foster and L. Whitelaw, BMJ, 321 (2000) 1608 (auditory hallucinations with tramadol)
- U. M. Stamer, F. Musshoff, M. Kobilay, B. Madea, A. Hoeft and F. Stuber, Clin. Pharmacol. Ther., 82 (2007) 41-47 (different CYP2D6 Genotypes and metabolism)
- W. Leppert, Pharmacology, 87 (2011)274–285 (metabolism of tramadol to O-desmethyltramadol by CYP2D6)
- A. Elkalioubie, D. Allorge, L. Robriquet, J.-F. Wiart, A. Garat, F. Broly and F. Fourrier, Eur. J. Clin. Pharmacol., 67 (2011) 855–858 (ultrarapid metabolism of tramadol)
- C. F. Samer, K. I. Lorenzini, V. Rollason, Y. Daali and J. A. Desmeules, Molecular Diagnosis & Therapy, 17 (2013), 165–84 (variations in tramadol response)
Tramadol abuse
- M. K. Wedge, Can. Pharm. J., 142 (2009) 71-73. (tramadol and antidepressants)
- ACMD advice on O-desmethyltramadol
- Y. Progler, J. Res. Med. Sci., 15 (2010) 185-188 (Tramadol abuse and smuggling into Gaza)
- M. M. Fawzi, Egypt. J. Forensic Sci., 1 (2011) 99–102 (Tramadol abuse in Egypt)
- I. Giraudon, K. Lowitz, P. I. Dargan, D. M. Wood and R. C. Dart, Br. J. Clin. Pharmacol., 76, (2013) 823–824 (prescription opioid abuse in the UK)
- C. Stannard, BMJ, 347 (2013) f5108 (tramadol problem)
- N. Hawkes, BMJ, 347 (2013) f5336 (deaths from tramadol and legal highs)
- A. Winstock, J. Bell and R. Borschmann, BMJ 347 (2013) f5599 (monitoring Tramadol abuse)
- S. H. Park, R. C. Wackernah and G. L. Stimmel, J. Pharm. Pract., 27 (2014) 71-78.
- Unemployment in Gaza and Tramadol addiction.
Tramadol in “highs”
- T. Arndt, U. Claussen, B. Güssregen, S. Schröfel, B. Stürzer, A. Werle and G. Wolf, For. Sci. Int., 208 (2011) 47-52. (Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer)
- R. Kronstrand, M. Roman, G. Thelander and A. Eriksson, J. Anal. Toxicol., 35 (2011) 242–247. (fatalities from mitragynine and O-desmethyltramadol combinations in Krypton herbal mixture)
- C. D. Rosenbaum, S. P. Carreiro and K. M. Babu, J. Med. Toxicol., 8 (2012) 15-32 (review of herbal marijuana alternatives, including Kratom)
Tramadol in cycling
- Michael Barry, “Shadows on the Road: Life at the Heart of the Peloton, from US Postal to Team Sky”, Faber 2014.
- Chris Froome, “The Climb: The Autobiography”, Viking, 2014.
- http://cyclingtips.com.au/2014/05/wada-proposes-tramadol-remains-a-monitored-rather-than-a-banned-substance-in-2015/
- Tramadol blamed for crashes
- Tramadol use for pain in cycling
| EP0778262A2 * | 19 Nov 1996 | 11 Jun 1997 | Chemagis Ltd. | Process for the purification of (RR-SS)-2-dimethyl-aminomethyl-1-(3-methoxyphenyl)cyclohexanol and its salts |
| EP0787715A1 * | 21 Dec 1996 | 6 Aug 1997 | Grünenthal GmbH | Process for the optical resolution of tramadol |
| EP0831082A1 * | 19 Aug 1997 | 25 Mar 1998 | Chemagis Ltd. | Process for the purification of (RR-SS)-2-dimethylaminomethyl-1-(3-methoxyphenyl)cyclohexanol hydrochloride |
| US5414129 * | 8 Sep 1993 | 9 May 1995 | Chemagis, Ltd. | Process for the purification of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol and its salts |
| Reference | ||
|---|---|---|
| 1 | * | CHEMICAL ABSTRACTS, vol. 126, no. 26, 30 June 1997 Columbus, Ohio, US; abstract no. 343383v, page 597; column 1; XP002079824 & IL 103 096 A (CHEMAGIS LTD) 5 December 1996 |
| 2 | * | CHEMICAL ABSTRACTS, vol. 127, no. 20, 17 November 1997 Columbus, Ohio, US; abstract no. 278028n, QIAO, BEN-ZHI ET AL.: “Synthesis and structure of 1-(m-methoxyphenyl)-2-(dimethylaminomethyl )cyclohexanol.” page 683; column 2; XP002079825 & GAODENG XUEXIAO HUAXUE XUEBAO , vol. 18, no. 6, 1997, pages 902-905, |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO1999036389A1 * | 14 Jan 1999 | 22 Jul 1999 | Nicholas Archer | Purification of tramadol |
| WO2000078705A1 * | 22 Jun 1999 | 28 Dec 2000 | Bernhard Akteries | Method for separating the diastereomer bases of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)-cyclohexanol |
| WO2003078380A2 * | 20 Mar 2003 | 25 Sep 2003 | Shahid Akhtar Ansari | Process for preparing tramadol hydrochloride and/or tramadol momohydrate |
| WO2004020390A1 * | 7 Aug 2003 | 11 Mar 2004 | Bernhard Akteries | Method for the production of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol |
| EP1346978A1 * | 21 Mar 2002 | 24 Sep 2003 | Jubilant Organosys Limited | Process for preparing tramadol hydrochloride and/or tramadol monohydrate |
| US6521792 * | 21 Dec 2001 | 18 Feb 2003 | Gruenenthal Gmbh | Process for separating the diastereomeric bases of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)-cylohexanol |
| US6649783 | 3 Oct 2001 | 18 Nov 2003 | Euro-Celtique, S.A. | Synthesis of (+/-)-2-((dimethylamino)methyl)-1-(aryl)cyclohexanols |
| US6784319 | 15 Sep 2003 | 31 Aug 2004 | Euro-Celtique, S.A. | Synthesis of (±)-2-((dimethylamino)methyl)-1-(aryl)cyclohexanols |
| US7030276 | 9 Feb 2005 | 18 Apr 2006 | Gruenenthal Gmbh | Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol |
| US8221792 | 7 Jul 2006 | 17 Jul 2012 | Farnam Companies, Inc. | Sustained release pharmaceutical compositions for highly water soluble drugs |
| EP0940385A1 * | Mar 3, 1999 | Sep 8, 1999 | Dinamite Dipharma S.p.A. | Process for the separation of the (RR,SS)-2-(dimethylamino)methyl-1-(3-methoxyphenyl)-cyclohexanol isomer from the (RS,SR) isomer by selective precipitation |
| WO1999003820A1 * | Jun 26, 1998 | Jan 28, 1999 | Nikolopoulos Angelo | Tramadol, salts thereof and process for their preparation |
| WO1999036390A1 * | Jan 14, 1999 | Jul 22, 1999 | Nicholas Archer | Purification of tramadol |
| WO2000078705A1 * | Jun 22, 1999 | Dec 28, 2000 | Bernhard Akteries | Method for separating the diastereomer bases of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)-cyclohexanol |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US7470816 | Nov 13, 2006 | Dec 30, 2008 | Ipac Laboratories Limited | Tramadol recovery process |
| US3652589 * | 27 Jul 1967 | 28 Mar 1972 | Gruenenthal Chemie | 1-(m-substituted phenyl)-2-aminomethyl cyclohexanols |
| US5414129 * | 8 Sep 1993 | 9 May 1995 | Chemagis, Ltd. | Process for the purification of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol and its salts |
| EP0778262A2 * | 19 Nov 1996 | 11 Jun 1997 | Chemagis Ltd. | Process for the purification of (RR-SS)-2-dimethyl-aminomethyl-1-(3-methoxyphenyl)cyclohexanol and its salts |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US6828345 * | 31 Mar 2003 | 7 Dec 2004 | Gruenenthal Gmbh | O-substituted 6-methyltramadol derivatives |
| US7030276 | 9 Feb 2005 | 18 Apr 2006 | Gruenenthal Gmbh | Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol |
| US7470816 | 13 Nov 2006 | 30 Dec 2008 | Ipac Laboratories Limited | Tramadol recovery process |
| US20050215821 * | 9 Feb 2005 | 29 Sep 2005 | Gruenenthal Gmbh | Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol |
| US20070112074 * | 13 Nov 2006 | 17 May 2007 | Ashok Kumar | Tramadol recovery process |
| EP0778262A2 * | Nov 19, 1996 | Jun 11, 1997 | Chemagis Ltd. | Process for the purification of (RR-SS)-2-dimethyl-aminomethyl-1-(3-methoxyphenyl)cyclohexanol and its salts |
| US3652589 * | Jul 27, 1967 | Mar 28, 1972 | Gruenenthal Chemie | 1-(m-substituted phenyl)-2-aminomethyl cyclohexanols |
| US5414129 * | Sep 8, 1993 | May 9, 1995 | Chemagis, Ltd. | Process for the purification of 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol and its salts |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| EP0940385A1 * | Mar 3, 1999 | Sep 8, 1999 | Dinamite Dipharma S.p.A. | Process for the separation of the (RR,SS)-2-(dimethylamino)methyl-1-(3-methoxyphenyl)-cyclohexanol isomer from the (RS,SR) isomer by selective precipitation |
| DE10218862A1 * | Apr 26, 2002 | Nov 6, 2003 | Gruenenthal Gmbh | Verfahren zur Chlorierung tertiärer Alkohole |
| US6169205 | Mar 4, 1999 | Jan 2, 2001 | Dipharma S.P.A. | Process for the purification of (RR,SS)-2-(dimethylamino) methyl-1-(3-methoxyphenyl)-cyclohexanol from (RS,SR)-2-(dimethylamino)methyl-1-(3-methoxyphenyl) cyclohexanol |
| US6469213 | Jan 14, 2000 | Oct 22, 2002 | Russinsky Limited | Tramadol, salts thereof and process for their preparation |
| US6649783 | Oct 3, 2001 | Nov 18, 2003 | Euro-Celtique, S.A. | Synthesis of (+/-)-2-((dimethylamino)methyl)-1-(aryl)cyclohexanols |
| US6784319 | Sep 15, 2003 | Aug 31, 2004 | Euro-Celtique, S.A. | Synthesis of (±)-2-((dimethylamino)methyl)-1-(aryl)cyclohexanols |
| US7235693 | Oct 26, 2004 | Jun 26, 2007 | Gruenenthal Gmbh | Process for chlorinating tertiary alcohols |
| US7470816 | Nov 13, 2006 | Dec 30, 2008 | Ipac Laboratories Limited | Tramadol recovery process |
| WO1999003820A1 * | Jun 26, 1998 | Jan 28, 1999 | Nikolopoulos Angelo | Tramadol, salts thereof and process for their preparation |
| Systematic (IUPAC) name | |
|---|---|
|
2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol
|
|
| Clinical data | |
| Trade names | Ryzolt, Tramal, Ultram |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a695011 |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Dependence liability |
Present[1] |
| Routes of administration |
Oral, IV, IM, rectal |
| Pharmacokinetic data | |
| Bioavailability | 70–75% (oral), 77% (rectal), 100% (IM)[2] |
| Protein binding | 20%[3] |
| Metabolism | Liver-mediated demethylation andglucuronidation via CYP2D6 &CYP3A4[2][3] |
| Biological half-life | 6.3 ± 1.4 hr[3] |
| Excretion | Urine (95%)[4] |
| Identifiers | |
| CAS Registry Number | 27203-92-5  |
| ATC code | N02AX02 |
| PubChem | CID: 33741 |
| DrugBank | DB00193  |
| ChemSpider | 31105  |
| UNII | 39J1LGJ30J  |
| KEGG | D08623  |
| ChEBI | CHEBI:9648  |
| ChEMBL | CHEMBL1066  |
| Chemical data | |
| Formula | C16H25NO2 |
| Molecular mass | 263.4 g/mol |
DMF
Tramadol hydrochloride..CEP
| Product Name | Country | Manufacture | Chemical formula | CAS # | CEP | DMF |
| Tramadol hydrochloride | India | IPCA Laboratories Ltd | C16H26ClNO2 | 22204-88-2 | R0-CEP 2008-189-Rev 00 | – – – |
| Tramadol hydrochloride | India | Dishman Pharmaceuticals and Chemicals Ltd. | C16H26ClNO2 | 22204-88-2 | R0-CEP 2003-148-Rev 01 | – – – |
| Tramadol hydrochloride | India | Sun Pharmaceutical Industries Ltd. | C16H26ClNO2 | 22204-88-2 | R1-CEP 2002-232-Rev 02 | – – – |
| Tramadol hydrochloride | China | CSPC OUYI PHARMACEUTICAL CO., LTD. | C16H26ClNO2 | 22204-88-2 | R0-CEP 2005-227-Rev 02 | – – – |
| Tramadol hydrochloride | India | JUBILANT LIFE SCIENCES LIMITED | C16H26ClNO2 | 22204-88-2 | R1-CEP 2002-199-Rev 03 | – – – |
| Tramadol hydrochloride | India | Cadila Pharmaceuticals Ltd. | C16H26ClNO2 | 22204-88-2 | R1-CEP 2004-098-Rev 01 | – – – |
| Tramadol hydrochloride | Germany | AREVIPHARMA GMBH | C16H26ClNO2 | 22204-88-2 | R0-CEP 2005-020-Rev 02 | – – – |
| Tramadol hydrochloride New process | India | Inogent Laboratories Private Limited | C16H26ClNO2 | 22204-88-2 | R0-CEP 2007-129-Rev 00 | – – – |
| Tramadol hydrochloride | India | SPIC Limited, Pharmaceuticals Division | C16H26ClNO2 | 22204-88-2 | R0-CEP 2004-245-Rev 00 | – – – |
| Tramadol hydrochloride | Switzerland | Cilag AG CH | C16H26ClNO2 | 22204-88-2 | R0-CEP 2006-262-Rev 00 | – – – |
| Tramadol hydrochloride | Italy | Dipharma Francis S.r.l. | C16H26ClNO2 | 22204-88-2 | R0-CEP 2002-105-Rev 01 | – – – |
| Tramadol hydrochloride | Israel | Chemagis Ltd | C16H26ClNO2 | 22204-88-2 | R1-CEP 2003-146-Rev 00 | – – – |
| Tramadol hydrochloride | India | Wanbury LTD | C16H26ClNO2 | 22204-88-2 | R0-CEP 2005-151-Rev 01 | – – – |
| Tramadol hydrochloride | Germany | Excella GmbH | C16H26ClNO2 | 22204-88-2 | R0-CEP 2003-137-Rev 02 | – – – |
| Tramadol hydrochloride | Switzerland | Proto Chemicals AG | C16H26ClNO2 | 22204-88-2 | R1-CEP 2002-204-Rev 01 | – – – |
| Tramadol hydrochloride | Czech Republic | ZENTIVA K.S. | C16H26ClNO2 | 22204-88-2 | R0-CEP 2009-214-Rev 00 | – – – |
| Tramadol hydrochloride | United States | Noramco, Inc. | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | Germany | GRUNENTHAL GMBH | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | Ireland | IROTEC LABORATORIES | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | Switzerland | HELSINN CHEMICALS SA | C16H26ClNO2 | 22204-88-2 | US – – | |
| TRAMADOL HYDROCHLORIDE | Israel | Chemagis Ltd | US – – | |||
| Tramadol hydrochloride | Slovakia | “ZENTIVA, A.S.” | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | United States | WYCKOFF CHEMICAL CO INC | C16H26ClNO2 | 22204-88-2 | US – – | |
| TRAMADOL HYDROCHLORIDE | Germany | Excella GmbH | US – – | |||
| Tramadol hydrochloride USP (BULK) | China | SHIJIAZHUANG PHARMACEUTICAL GROUP CO LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| TRAMADOL HYDROCHLORIDE | Germany | EVONIK DEGUSSA GMBH | US – – | |||
| Tramadol hydrochloride | Italy | RECORDATI S.p.A. | C16H26ClNO2 | 22204-88-2 | US – – | |
| TRAMADOL HYDROCHLORIDE | India | Dishman Pharmaceuticals and Chemicals Ltd. | US – – | |||
| TRAMADOL HYDROCHLORIDE | India | Sun Pharmaceutical Industries Ltd. | US – – | |||
| TRAMADOL HYDROCHLORIDE | India | Cadila Pharmaceuticals Ltd. | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | Switzerland | PROTO CHEMICALS AG | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride EP | India | DAI ICHI KARKARIA LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | India | PEARL ORGANICS LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride EP | China | SHIJIAZHUANG PHARMACEUTICAL GROUP HUASHENG PHARMA CO LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| TRAMADOL HYDROCHLORIDE | India | JUBILANT LIFE SCIENCES LIMITED | US – – | |||
| TRAMADOL HYDROCHLORIDE | Germany | AREVIPHARMA GMBH | US – – | |||
| Tramadol hydrochloride | India | TONIRA PHARMA LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | India | INOGENT LABORATORIES PRIVATE LIMITED | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | China | ZHEJIANG HISOAR PHARMACEUTICAL CO LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| TRAMADOL HYDROCHLORIDE | India | IPCA Laboratories Ltd | US – – | |||
| Tramadol hydrochloride | China | HEBEI ZHONGSHENG PHARMACEUTICAL CO LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride BP | India | KAMUD DRUGS PVT LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | India | Raks Pharma Pvt Ltd. | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride USP n/sterile | India | Aurobindo Pharma Ltd. | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride (YT3 PROCESS) | India | IPCA Laboratories Ltd | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | India | PIRAMAL HEALTHCARE LTD | C16H26ClNO2 | 22204-88-2 | US – – | |
| Tramadol hydrochloride | India | Asence Pharma Private Ltd | C16H26ClNO2 | 22204-88-2 | – – – |
SYNOPSIS FOR M. PHARM DISSERTATION
Formulated and Optimized Hydrodynamically balanced Oral Controlled release Bioadhesive
Tablets of Tramadol Hydrochloride using different polymers like Carbopol 971P (CP) and …
////////TRAMADOL,
Filed under: Uncategorized Tagged: TRAMADOL