

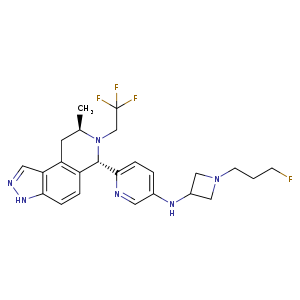
Camizestrant, AZD 9833
AZ 14066724
PHASE 2
CAS: 2222844-89-3
Chemical Formula: C24H28F4N6
Exact Mass: 476.2312
Molecular Weight: 476.5236
Elemental Analysis: C, 60.49; H, 5.92; F, 15.95; N, 17.64
N-(1-(3-fluoropropyl)azetidin-3-yl)-6-((6S,8R)-8-methyl-7-(2,2,2-trifluoroethyl)-6,7,8,9-tetrahydro-3H-pyrazolo[4,3-f]isoquinolin-6-yl)pyridin-3-amine
- AZ14066724
- AZD-9833
- AZD9833
- Camizestrant
- UNII-JUP57A8EPZ
- WHO 11592
- OriginatorAstraZeneca
- ClassAmines; Antineoplastics; Azetidines; Fluorinated hydrocarbons; Isoquinolines; Pyrazolones; Pyridines; Small molecules
- Mechanism of ActionSelective estrogen receptor degraders
- Phase IIIBreast cancer
- 13 Jun 2022AstraZeneca initiates a phase I drug-drug interaction trial of AZD 9833 Healthy postmenopausal female volunteers, in USA (NCT05438303)
- 10 Jun 2022AstraZeneca and Quotient Sciences complete the phase I QSC205863 trial in Breast cancer (In volunteers) in United Kingdom (PO, Liquid) (NCT05364255)
- 03 Jun 2022Safety, efficacy and pharmacokinetics data from the phase I SERENA 1 trial for Breast cancer presented at the 58th Annual Meeting of the American Society of Clinical Oncology (ASCO-2022)
- Mechanism:selective estrogen receptor degrader
- Area under investigation:estrogen receptor +ve breast cancer
- Date commenced phase:Q1 2019
- Estimated Filing Acceptance:
- CountryDateUS: EU: Japan: China:
AZD9833 is an orally available selective estrogen receptor degrader (SERD), with potential antineoplastic activity. Upon administration, SERD AZD9833 binds to the estrogen receptor (ER) and induces a conformational change that results in the degradation of the receptor. This prevents ER-mediated signaling and inhibits the growth and survival of ER-expressing cancer cells
Camizestrant is an orally available selective estrogen receptor degrader (SERD), with potential antineoplastic activity. Upon administration, camizestrant binds to the estrogen receptor (ER) and induces a conformational change that results in the degradation of the receptor. This prevents ER-mediated signaling and inhibits the growth and survival of ER-expressing cancer cells
SYN
https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0040-1719368
Discovery of AZD9833, a Potent and Orally Bioavailable Selective Estrogen Receptor Degrader and Antagonist J. Med. Chem. 2020, 63, 14530–14559, DOI: 10.1021/acs.jmedchem.0c01163.

SYN
doi: 10.1021/acs.jmedchem.0c01163.

aReagents and Conditions: (a) n-BuLi, THF, −78 oC to 0 oC, 1 h, then 4 N HCl/dioxane, RT, 1 h, 60%; (b) alkyl triflate, DIPEA, 1,4-dioxane, 90 oC, 63-74% or isobutyrylaldehyde, Na(OAc)3BH, THF, 0 oC, 56%; (c) benzophenone imine, Pd2dba3, Rac-BINAP, NaOtBu, toluene, 90 oC, then 1 N aq. HCl, 71-85%; (d) nBuLi, THF, −78 oC to 0 oC, 1 h, then 4 N HCl/dioxane, RT, 4 h; e) NH2OH, NH2OH.HCl, EtOH, reflux. 84% over 2 steps; (f) alkyl triflate, DIPEA, 1,4-dioxane, 90 oC, 44-100% or 1-fluorocyclopropane-1- carboxylic acid, HATU, Et3N, DMF, RT, 61%, then BH3.THF, THF, 65 oC, 82%.

[α]26 D -147 (c 2.3, MeOH); 1H NMR (500 MHz, DMSO-d6, 27 °C) 1.08 (d, J = 6.6 Hz, 3H), 1.64 (dp, J = 25.0, 6.3 Hz, 2H), 2.45 (t, J = 6.9 Hz, 2H), 2.73(t, J = 6.8 Hz, 2H), 2.84 (dd, J = 17.1, 8.2 Hz, 1H), 2.96 (dt, J = 19.6, 9.8 Hz, 1H), 3.07 (dd, J = 17.2, 4.6 Hz, 1H), 3.49 (m, 1H), 3.50 – 3.58 (m, 1H), 3.58 – 3.66 (m, 2H), 3.92 (h, J = 6.5 Hz, 1H), 4.44 (dtd, J = 47.4, 6.1, 1.3 Hz, 2H), 4.93 (s, 1H), 6.23 (d, J = 6.9 Hz, 1H), 6.80 (d, J = 8.6 Hz, 1H), 6.83 (dt, J = 8.8, 2.0 Hz, 1H), 6.97 (d, J = 8.5 Hz, 1H), 7.22 (d, J = 8.6 Hz, 1H), 7.73 (d, J = 2.8 Hz, 1H), 8.05 (d, J = 1.3 Hz, 1H), 12.97 (s, 1H); 13C NMR (125 MHz, DMSO-d6, 27 °C) 16.2, 28.2 (d, J = 19.4 Hz), 30.1, 43.0, 47.3, 48.7 (q, J = 30.1 Hz), 54.8 (d, J = 5.6 Hz), 61.3 (2C), 67.1, 82.0 (d, J = 161.3 Hz), 107.5, 119.0, 122.4, 123.7, 126.1, 126.2 (q, J = 278.5 Hz), 126.4, 127.5, 131.7, 132.9, 138.5, 142.3, 150.0; 19F NMR (376 MHz, DMSO-d6, 27 °C) -218.1 (1F), -69.7 (3F); m/z (ES+), [M+H]+ = 477, HRMS (ESI) (MH+ ); calcd, 477.2408; found, 477.2390




/////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
AZD9833 is selective oestrogen receptor degrader (SERD). It works by breaking down the site where oestrogen attaches to the cancer cell. This can help stop or slow the growth of hormone receptor breast cancer. Researchers think that AZD9833 with palbociclib might work better than anastrozole and palbociclib.
AZD9833 + palbociclib
The patients will receive AZD9833 (75 mg, PO, once daily) + palbociclib (PO, once daily, 125 mg for 21 consecutive days followed by 7 days off treatment) + anastrozole placebo (1 mg, PO, once daily)
SERENA-1: Study of AZD9833 Alone or in Combination in Women With Advanced Breast Cancer. (clinicaltrials.gov)…..https://veri.larvol.com/news/azd9833/drug
P1, N=305, Recruiting, AstraZeneca | Trial primary completion date: Dec 2022 –> Oct 2023
2 months ago
Trial primary completion date
|
HER-2 (Human epidermal growth factor receptor 2) • ER (Estrogen receptor) • PGR (Progesterone receptor)
|
HER-2 negative
Ibrance (palbociclib) • everolimus • Verzenio (abemaciclib) • capivasertib (AZD5363) • camizestrant (AZD9833)
| Description | Camizestrant (AZD-9833) is a potent and orally active estrogen receptor (ER) antagonist. Camizestrant is used for the study of ER+ HER2-advanced breast cancer[1]. |
|---|---|
| IC50 & Target | IC50: estrogen receptor (ER)[1] |
| In Vitro | Camizestrant is extracted from patent US20180111931A1, example 17[1].MCE has not independently confirmed the accuracy of these methods. They are for reference only. |
| In Vivo | Camizestrant (oral administration; 0.2-50 mg/kg; 20 days) exhibits anti-tumour efficacy as a dose-dependent manner in human parental MCF7 mice xenograft[1]. Camizestrant (oral administration; 0.8-40 mg/kg; 30 days) decreases tumor growth as a dose-dependent manner. It gives almost complete tumour growth inhibition at the doses >10 mg/kg in mice[1]. MCE has not independently confirmed the accuracy of these methods. They are for reference only.Animal Model:Human ESR1 mutant breast cancer patient derived xenograft with CTC174 cells in female NSG mice[1]Dosage:0.8 mg/kg, 3 mg/kg, 10 mg/kg, 20 mg/kg, 40 mg/kgAdministration:Oral administration; 30 days; once dailyResult:Inhibited tumor growth in a dose-dependent manner. |
| Clinical Trial | NCT NumberSponsorConditionStart DatePhaseNCT04711252AstraZenecaER-Positive HER2-Negative Breast CancerJanuary 28, 2021Phase 3NCT04964934AstraZenecaER-Positive HER2-Negative Breast CancerJune 30, 2021Phase 3NCT04214288AstraZenecaAdvanced ER-Positive HER2-Negative Breast CancerApril 22, 2020Phase 2NCT04588298AstraZenecaHER2-negative Breast CancerNovember 2, 2020Phase 2NCT04541433AstraZenecaER&addition; HER2- Advanced Breast CancerSeptember 29, 2020Phase 1NCT03616587AstraZenecaER&addition; HER2- Advanced Breast CancerOctober 11, 2018Phase 1NCT04546347AstraZeneca|Quotient SciencesHealthy VolunteersSeptember 17, 2020Phase 1NCT04818632AstraZenecaER&addition;, HER2-, Metastatic Breast CancerOctober 11, 2021Phase 1 |
////////////Camizestrant, AZD 9833, AZ 14066724, UNII-JUP57A8EPZ, WHO 11592, PHASE 2, ASTRA ZENECA, CANCER
C[C@@H]1CC2=C3C(NN=C3)=CC=C2[C@@H](C4=NC=C(NC5CN(CCCF)C5)C=C4)N1CC(F)(F)F
