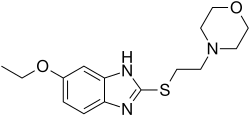

Fabomotizole
Afobazole
- Molecular FormulaC15H21N3O2S
- Average mass307.411 Da
0F8K1X115C
173352-21-1[RN], 173352-21-1 (free base) 173352-39-1 (HCl) 189638-30-0 (2HCl)
1H-Benzimidazole, 6-ethoxy-2-[[2-(4-morpholinyl)ethyl]thio]-
Obenoxazine, Afobazol, Afobazole, Aphobazole, Fabomotizole dihydrochloride, CM-346, CM346, CM 346,
фабомотизол[Russian][INN]
فابوموتيزول[Arabic][INN]
法莫替唑[Chinese][INN]

Fabomotizole dihydrochloride
CAS#: 189638-30-0 (2HCl)
Chemical Formula: C15H23Cl2N3O2S
Molecular Weight: 380.33
Fabomotizole (also known as Afobazole) is a selective non-benzodiazepine anxiolytic which was developed in Russia and launched in 2006. The drug is used for the treatment of wide range of diseases: generalized anxious disorders, neurasthenia, adaptation disorders, sleep disorders, for alleviation of withdrawal syndrome. According to the drug label (in Russian), its action is related to the interaction with sigma-1 receptors.
Fabomotizole (INN;[1] brand name Afobazole) is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions.[citation needed] Its mechanism of action remains poorly defined however, with GABAergic, NGF– and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors.[2][3][4][5][6] Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety.[7]
Experiments of mice have shown antimutagenic and antiteratogenic properties.[8]
Fabomotizole has found little clinical use outside Russia and has not been evaluated by the FDA.
PATENT
WO 9534304
https://patents.google.com/patent/WO1995034304A1/en

PAPER
European Journal of Medicinal Chemistry (2021), 211, 113110
https://www.sciencedirect.com/science/article/abs/pii/S0223523420310825?
A ligand-based virtual screening study to search for giardicidal compounds on a 6551 ChEMBL drugs database was carried out using molecular similarity. Three fingerprints implemented in MayaChemTools with different design and validated by ROC curves, were used. Twelve compounds were retrieved from this screening, from which, four representative compounds were selected to carry out biological assays. Whereas two compounds were commercially available, the additional two compounds were synthesized during the development of this work. The biological assays revealed that the compounds possess in vitro activity against five strains of Giardia intestinalis, each with different susceptibility/resistance rates to metronidazole, albendazole and nitazoxanide. Particularly, tenatoprazole showed the best effect against the WB and IMSS strains. Furthermore, fabomotizole, tenatoprazole and ipriflavone showed a higher activity against resistant strains than the reference drugs: metronidazole, albendazole and nitazoxanide.
Graphical abstract


///////////////////////////////////////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
| Clinical data | |
|---|---|
| Trade names | Afobazole |
| Other names | Fabomotizole |
| Routes of administration | Oral |
| ATC code | N05BX04 (WHO) |
| Legal status | |
| Legal status | US: Unscheduled Not FDA approved |
| Pharmacokinetic data | |
| Bioavailability | 43.64%, pronounced first-pass effect |
| Metabolism | extensive hepatic |
| Onset of action | 0.85±0.13 hours |
| Elimination half-life | 0.82±0,54 hours |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 173352-39-1 |
| PubChem CID | 9862937 |
| ChemSpider | 8038633 |
| UNII | HDO6HX6NZU |
| CompTox Dashboard (EPA) | DTXSID00169606 |
| Chemical and physical data | |
| Formula | C15H21N3O2S |
| Molar mass | 307.41 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |
References
- ^ “International Nonproprietary Names for Pharmaceutical Substances (INN)” (PDF). WHO Drug Information. 26 (1): 63. 2012. Retrieved 21 March 2015.
- ^ Neznamov, GG; Siuniakov, SA; Chumakov, DV; Bochkarev, VK; Seredenin, SB (2001). “Clinical study of the selective anxiolytic agent afobazol”. Eksperimental’naia i Klinicheskaia Farmakologiia. 64 (2): 15–9. PMID 11548440.
- ^ Silkina, IV; Gan’shina, TC; Seredin, SB; Mirzoian, RS (2005). “Gabaergic mechanism of cerebrovascular and neuroprotective effects of afobazole and picamilon”. Eksperimental’naia i Klinicheskaia Farmakologiia. 68 (1): 20–4. PMID 15786959.
- ^ Seredin, SB; Melkumian, DS; Val’dman, EA; Iarkova, MA; Seredina, TC; Voronin, MV; Lapitskaia, AS (2006). “Effects of afobazole on the BDNF content in brain structures of inbred mice with different phenotypes of emotional stress reaction”. Eksperimental’naia i Klinicheskaia Farmakologiia. 69 (3): 3–6. PMID 16878488.
- ^ Antipova, TA; Sapozhnikova, DS; Bakhtina, LIu; Seredenin, SB (2009). “Selective anxiolytic afobazole increases the content of BDNF and NGF in cultured hippocampal HT-22 line neurons”. Eksperimental’naia i Klinicheskaia Farmakologiia. 72 (1): 12–4. PMID 19334503.
- ^ Seredenin, SB; Antipova, TA; Voronin, MV; Kurchashova, SY; Kuimov, AN (2009). “Interaction of afobazole with sigma1-receptors”. Bulletin of Experimental Biology and Medicine. 148 (1): 42–4. doi:10.1007/s10517-009-0624-x. PMID 19902093. S2CID 37411324.
- ^ Medvedev, VE; Trosnova, AP; Dobrovol’skiĭ, AV (2007). “Psychopharmacotherapy of anxiety disorders in patients with cardio-vascular diseases: the use of aphobazole”. Zh Nevrol Psikhiatr Im S S Korsakova. 107 (7): 25–9. PMID 18379478.
- ^ Durnev AD, Zhanataev AK, Shreder OV, Seredenin SB (Jan–Feb 2009). “Antimutagenic and antiteratogenic properties of afobazole”. Eksp Klin Farmakol. 72 (1): 46–51. PMID 19334511.
//////////////Fabomotizole, Afobazole, фабомотизол , فابوموتيزول , 法莫替唑 , Obenoxazine, Afobazol, Afobazole, Aphobazole, Fabomotizole dihydrochloride, CM-346, CM346, CM 346,
CCOc1ccc2c(c1)[nH]c(n2)SCCN3CCOCC3.Cl.Cl
