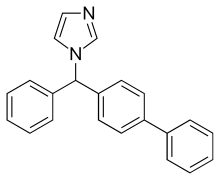
BIFONAZOLE
- Molecular FormulaC22H18N2
- Average mass310.392 Da
(±)-1-(p,a-Diphenylbenzyl)imidazole
(±)-Bifonazole
1-([1,1′-Biphenyl]-4-ylphenylmethyl)-1H-imidazole
1-(p,α-Diphenylbenzyl)imidazole
262-336-6[EINECS]
4887
60628-96-8[RN]
бифоназол
بيفونازول
联苯苄唑
- BAY H 4502
- BAY-H-4502
Bifonazole
CAS Registry Number: 60628-96-8
CAS Name: 1-([1,1¢-Biphenyl]-4-ylphenylmethyl)-1H-imidazole
Additional Names: (±)-1-(p,a-diphenylbenzyl)imidazole
Manufacturers’ Codes: Bay h 4502
Trademarks: Amycor (Lipha); Azolmen (Menarini); Bedriol (Andromaco); Mycospor (Bayer); Mycosporan (Bayer)
Molecular Formula: C22H18N2, Molecular Weight: 310.39
Percent Composition: C 85.13%, H 5.85%, N 9.03%
Literature References: Antimycotic deriv of imidazole. Prepn: E. Regel et al.,DE2461406; eidem,US4118487 (1976, 1978 both to Bayer). Series of articles on in vitro and in vivo antimycotic efficacy, microscopic studies, pharmacokinetics, efficacy in dermatomycoses and comparison with clotrimazole and miconazole, q.q.v.:Arzneim.-Forsch.33, 517-551, 745-754 (1983). Toxicology: G. Schlüter, ibid. 739.
Properties: Crystals from acetonitrile, mp 142°. Very lipophilic. Sol in alcohols, DMF, DMSO. Soly in water at pH 6: <0.1 mg/100 ml. Stable in aq soln at pH 1-12. LD50 in male mice, rats (mg/kg): 2629, 2854 orally (Schlüter).
Melting point: mp 142°
Toxicity data: LD50 in male mice, rats (mg/kg): 2629, 2854 orally (Schlüter)
Therap-Cat: Antifungal.
Keywords: Antifungal (Synthetic); Imidazoles.
BrandsAmycor (Merck) / Azolmen (Menarini) / Bayclear Plus (Bayer) / Bifonol (Mayado Seiyaku) / Canespor (Bayer) / Canesten (Bayer) / Mycospor (Bayer)
Bifonazole (trade name Canespor among others[1]) is an imidazole antifungal drug used in form of ointments.
It was patented in 1974 and approved for medical use in 1983.[2] There are also combinations with carbamide for the treatment of onychomycosis.
Bifonazole is an azole antifungal drug used to treat fungal skin infections, such as dermatomycosis.
- Synonyms:Bifonazolum
- ATC:D01AC10
- MW:310.40 g/mol
- CAS-RN:60628-96-8
- InChI Key:OCAPBUJLXMYKEJ-UHFFFAOYSA-N
- InChI:InChI=1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H
- EINECS:262-336-6
- LD50:57 mg/kg (M, i.v.); 2629 mg/kg (M, p.o.);
63 mg/kg (R, i.v.); 1463 mg/kg (R, p.o.);
>500 mg/kg (dog, p.o.)
Derivatives
Monohydrochloride
- Formula:C22H18N2 • HCl
- MW:346.86 g/mol
- CAS-RN:60629-09-6
Sulfate
- Formula:C22H18N2 • xH2O4S
- MW:unspecified
- CAS-RN:60629-08-5
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 98-88-4 | C7H5ClO | benzoyl chloride | Benzoyl chloride |
| 92-52-4 | C12H10 | biphenyl | 1,1′-Biphenyl |
| 7515-73-3 | C19H15Cl | (±)-4-(chlorophenylmethyl)biphenyl | 1,1′-Biphenyl, 4-(chlorophenylmethyl)- |
| 288-32-4 | C3H4N2 | imidazole | 1H-Imidazole |
SYN
Synthesis Reference
Regal, E., Draber, W., Buchel, K.H.and Plempel, M.; U.S. Patent 4,118,487; October 3,1978; assigned to Bayer A.G.
SYN

SYN
(CAS NO.: ), with its systematic name of , 1-(alpha-(4-biphenylyl)benzyl)-, could be produced through many synthetic methods.
Following is one of the synthesis routes: (I) could be reduced with NaBH4 in ethanol to produce 4-phenylbenzhydrol (II), and the yielding product is then condensed with imidazole (III) in the presence of SOCl2 in acetonitrile.

PAT
https://patents.google.com/patent/DE10332684B3/en
- The The present invention relates to a process for the preparation of Bifonazole (1- [biphenyl-4-yl (phenyl) methyl] -1H-imidazole) by reacting 1-biphenyl-4-yl (phenyl) methanol with a chlorinating reagent in cyclohexane and subsequent coupling with imidazole.
- [0002]The compound bifonazole (1- [biphenyl-4-yl (phenyl) methyl] -1H-imidazole) is off DE-A 2 461 406 known and corresponds to the formula (I). Due to its antifungal activity, it can be used as an agent for the treatment of fungal diseases.
- [0003]Various methods for preparing this compound are known. So describes DE-A 2 461 406 the synthesis (process 1) of bifonazole (Example 1) starting from biphenyl-4-yl (phenyl) methanol by reaction with imidazole and thionyl chloride in acetonitrile with a yield of only 56% of theory. An alternative synthesis described therein (process 2) starting from 4- [chloro (phenyl) methyl] biphenyl, which is prepared from biphenyl-4-yl (phenyl) methanol by reaction with thionyl chloride in toluene, by reaction with trimethylsilylimidazole bifonazole provides only in a yield of 52% of theory.
- [0004]ES-A 2 024 363 describes also starting from 4- [chloro (phenyl) methyl] biphenyl, which is prepared from biphenyl-4-yl (phenyl) methanol by reaction with hydrogen chloride in acetonitrile, by reaction with imidazole in acetonitrile using a phase transfer catalyst, the synthesis (method 3) of bifonazole.
- [0005]AT-B 396 931 describes the preparation (method 4) of bifonazole by means of reductive amination of biphenyl-4-yl (phenyl) methanone with imidazole and formic acid. However, this requires high reaction temperatures (220 ° C.) and long reaction times. DE-A 3 538 873 describes a comparable process (process 5) with the additional use of p-toluenesulfonic acid, wherein the reaction temperature is 180 ° C.
- [0006]This in ES 539 345 described method (method 6) for the preparation of bifonazole involves a Gringard reaction between 4-biphenylmagnesium bromide and benzoylated imidazole. Finally, it is tosylated and reduced to bifonazole.
- [0007]ES 549 793 describes the synthesis (method 7) of bifonazole starting from a cyclocondensation between biphenyl-4-yl (phenyl) methylamine, 2-chloro-1-aminoethane and ethyl orthoacetate. The final dehydrogenation is carried out by reaction with 2,3-dichloro-5,6-dicyano-p-benzoquinone in benzene.
- [0008]All known processes have various disadvantages which are particularly unfavorable in the preparation of the compound of the formula (I) on an industrial scale. The solvents used in processes 1 and 2 acetonitrile and toluene are of concern to health. Their use should be avoided in the manufacture of active ingredients used in medicines. By using toluene in process 2, chlorination to give 4- [chloro (phenyl) methyl] biphenyl also produces a toluene-specific, undesired by-product which can only be removed incompletely and thus deteriorates the product quality. The yield is unsatisfactory in both processes. A significant disadvantage of method 3 is, in addition to the use of acetonitrile as solvent, the use of a phase transfer catalyst, which is difficult to separate from the product during work-up. Methods 4 and 5 both operate at very high temperatures and are therefore disadvantageous in a technical use due to the energy consumption and the potential hazard. In method 6, the use of the Gringard reagent is disadvantageous, since this must be produced under considerable safety expense and difficult to handle on an industrial scale. Disadvantage in process 7 is the use of the very toxic compounds 2,3-dichloro-5,6-dicyano-p-benzoquinone and benzene. Their use should be avoided especially in the production of active ingredients used in pharmaceuticals
- Embodiment:
- Synthesis of bifonazole (1- [Biphenyl-4-yl (phenyl) methyl] -1H-imidazole)
- 1st step: 4- [chloro (phenyl) methyl] biphenyl (III)
- [0038]140 g (0.54 mol) dry (water content <0.3%) biphenyl-4-yl (phenyl) methanol (II) are suspended in 1550 ml of cyclohexane and treated with 90 g (0.76 mol) thionyl chloride at a temperature of 50 to 55 ° C added. The reaction mixture is stirred for 0.5 h at a temperature of 50 to 55 ° C stirred. Subsequently, in the Vacuum (<100 mbar) Distilled off thionyl chloride and cyclohexane. A distillation bottoms containing 4- [chloro (phenyl) methyl] biphenyl remains.
- 2nd step: 1- [biphenyl-4-yl (phenyl) methyl] -1H-imidazole (Bifonazole)
- [0039]162 g (2.4 mol) of imidazole are suspended in 1350 ml of acetone and dissolved at 50 ° C. This solution is added to the distillation bottoms from step 1 containing 4- [chloro (phenyl) methyl] biphenyl (III). The reaction mixture is heated at reflux for 3 h. After cooling, the reaction solution is mixed with 2 g of activated carbon and 2 g of bleaching earth at a temperature of 50 to 55 ° C, stirred for 0.5 h and filtered. The filtrate is cooled to about 0 ° C. The title compound crystallizes by addition of seed crystals, is filtered off and washed with a mixture of acetone / water (1: 1). For recrystallization, the product is dissolved in 1250 ml of isopropanol, treated with 0.5 g of activated charcoal and 0.5 g of bleaching earth, heated to reflux and filtered hot. The filtrate is cooled to 10 ° C. The title compound crystallizes out by addition of seed crystals, is filtered off, washed with isopropanol and dried. The yield is 101 g (61.9% of theory). The purity of the product is 98.68% by weight.
Melting point: 142 ° C - Comparative method:
- [0040]In the comparative method, instead of cyclohexane, toluene is used as solvent in step 1 as in DE-A 2 461 406 described. Step 2 is performed as described above. 1- [biphenyl-4-yl (phenyl) methyl] -1H-imidazole (bifonazole) is obtained in a purity of 97.66% by weight.
///////////////////////////////////////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Adverse effects
The most common side effect is a burning sensation at the application site. Other reactions, such as itching, eczema or skin dryness, are rare.[3] Bifonazole is a potent aromatase inhibitor in vitro.[4][5]
Pharmacology
Mechanism of action
Bifonazole has a dual mode of action. It inhibits fungal ergosterol biosynthesis at two points, via transformation of 24-methylendihydrolanosterol to desmethylsterol, together with inhibition of HMG-CoA. This enables fungicidal properties against dermatophytes and distinguishes bifonazole from other antifungal drugs.[3][6]
Pharmacokinetics
Six hours after application, bifonazole concentrations range from 1000 µg/cm³ in the stratum corneum to 5 µg/cm³ in the papillary dermis.[3]
References
- ^ International Drug Names: Bifonazole.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 502. ISBN 9783527607495.
- ^ Jump up to:a b c Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Canesten Bifonazol-Creme.
- ^ Trösken ER, Fischer K, Völkel W, Lutz WK (February 2006). “Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation”. Toxicology. 219 (1–3): 33–40. doi:10.1016/j.tox.2005.10.020. PMID 16330141.
- ^ Egbuta C, Lo J, Ghosh D (December 2014). “Mechanism of inhibition of estrogen biosynthesis by azole fungicides”. Endocrinology. 155 (12): 4622–8. doi:10.1210/en.2014-1561. PMC 4239419. PMID 25243857.
- ^ Berg D, Regel E, Harenberg HE, Plempel M (1984). “Bifonazole and clotrimazole. Their mode of action and the possible reason for the fungicidal behaviour of bifonazole”. Arzneimittel-Forschung. 34 (2): 139–46. PMID 6372801.
Further reading
- Lackner TE, Clissold SP (August 1989). “Bifonazole. A review of its antimicrobial activity and therapeutic use in superficial mycoses”. Drugs. 38 (2): 204–25. doi:10.2165/00003495-198938020-00004. PMID 2670516.
| Clinical data | |
|---|---|
| Trade names | Canespor, many others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical |
| ATC code | D01AC10 (WHO) |
| Legal status | |
| Legal status | In general: Over-the-counter (OTC) |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 60628-96-8 |
| PubChem CID | 2378 |
| DrugBank | DB04794 |
| ChemSpider | 2287 |
| UNII | QYJ305Z91O |
| KEGG | D01775 |
| ChEBI | CHEBI:31286 |
| ChEMBL | ChEMBL277535 |
| CompTox Dashboard (EPA) | DTXSID9045631 |
| ECHA InfoCard | 100.056.651 |
| Chemical and physical data | |
| Formula | C22H18N2 |
| Molar mass | 310.400 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| Chirality | Racemic mixture |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |
///////////BIFONAZOLE, бифоназол , بيفونازول , 联苯苄唑 , BAY H 4502, BAY-H-4502
C1=CN(C=N1)C(C1=CC=CC=C1)C1=CC=C(C=C1)C1=CC=CC=C1
