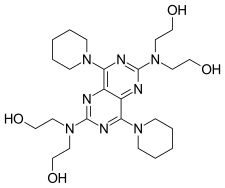
Dipyridamole
- Molecular FormulaC24H40N8O4
- Average mass504.626 Da
2,2′,2”,2”’-{[4,8-Di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl]dinitrilo}tetraethanol
200-374-7[EINECS]
58-32-2[RN]
Ethanol, 2,2′,2”,2”’-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakis-
дипиридамол [Russian] [INN]
ديبيريدامول [Arabic] [INN]
双嘧达莫 [Chinese] [INN]
0068373 [Beilstein]
DipyridamoleCAS Registry Number: 58-32-2
CAS Name: 2,2¢,2¢¢,2¢¢¢-[(4,8-Di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakisethanol
Additional Names: 2,6-bis(diethanolamino)-4,8-dipiperidinopyrimido-[5,4-d]pyrimidine
Manufacturers’ Codes: NSC-515776; RA-8
Trademarks: Anginal (Yamanouchi); Cardoxin (RAFA); Cleridium (Marcofina); Coridil (Delalande); Coronarine (NEGMA); Curantyl (Berlin-Chemie); Dipyridan (Hokuriku); Gulliostin (Taiyo); Natyl (Interdelta); Peridamol (Boehringer, Ing.); Persantine (Boehringer, Ing.); Piroan (Towa Yakuhin); Prandiol (Bottu); Protangix (Lefrancq)
Molecular Formula: C24H40N8O4
Molecular Weight: 504.63
Percent Composition: C 57.12%, H 7.99%, N 22.21%, O 12.68%
Literature References: Phosphodiesterase inhibitor that reduces platelet aggregation; also acts as a coronary vasodilator. Prepn: GB807826; F. G. Fischer, et al.,US3031450 (1959, 1962 both to Thomae). Activity studies: Saraf, Seth, Indian J. Physiol. Pharmacol.15, 135 (1971). Toxicological study: F. Takenaka et al.,Arzneim.-Forsch.22, 892 (1972). Symposium on pharmacology and clinical experience as antithrombotic: Thromb. Res.60, Suppl. 12, 1-99 (1990). Review of use as pharmacological stress agent in echocardiography: M. B. Buchalter et al.,Postgrad. Med. J.66, 531-535 (1990); in 201Tl cardiac imaging: S. G. Beer et al.,Am. J. Cardiol.67, Suppl., 18D-26D (1991).
Properties: Deep yellow needles from ethyl acetate, mp 163°. Bitter taste. Slightly sol in H2O; sol in dil acid having a pH of 3.3 or below; very sol in methanol, ethanol, chloroform; not too sol in acetone, benzene, ethyl acetate. Solns are yellow and show strong blue-green fluorescence. LD50 in rats: 8.4 g/kg orally; 208 mg/kg i.v. (Takenaka).Melting point: mp 163°
Toxicity data: LD50 in rats: 8.4 g/kg orally; 208 mg/kg i.v. (Takenaka)
Derivative Type: Combination with aspirin
Trademarks: Aggrenox (Boehringer, Ing.)
Literature References: Review of pharmacology and clinical efficacy in secondary prevention of stroke: P. S. Hervey, K. L. Goa, Drugs58, 469-475 (1999).
Therap-Cat: Antithrombotic; diagnostic aid (cardiac stress testing).
Keywords: Antithrombotic; Diagnostic Aid; Phosphodiesterase Inhibitor.
Dipyridamole (trademarked as Persantine and others) is a nucleoside transport inhibitor and a PDE3 inhibitor medication that inhibits blood clot formation[3] when given chronically and causes blood vessel dilation when given at high doses over a short time.
PATENT
https://patents.google.com/patent/WO2011151640A1/enDipyridamole, represented by structural formula (I), possesses platelet aggregation inhibiting, anti-thrombotic and vasodilator properties and it is marketed as an anti-platelet therapy for the treatment and prevention of disorders such as thrombo-embolisms.

A process for the preparation of dipyridamole, disclosed in patent US 3031450, involves the reaction of 2,6-dichloro-4,8-dipiperidino-pyrimido(5,4-d)pyrimidine with diethanolamine (see Scheme 1). The preparation of 2,6-dichloro-4,8-dipiperidino- pyrimido(5,4-d)pyrimidine is also reported in US 3031450 and is incorporated herein by reference. The reaction to prepare dipyridamole does not employ an additional reaction solvent and is a neat mixture of the two reactants carried out at a very high temperature of 190 to 195°C. The process also involves a cumbersome work-up to isolate dipyridamole, since the crude product obtained is a pasty mass which needs decantation of the mother liquor and further purification. This decantation process is not practical on commercial scale.

2,6-dichloro-4,8-dipiperidino- dipyridamole (Ί) pyflmido(5,4-d)pyrimidineScheme 1A similar process for the production of dipyridamole is described in patent DD 117456 wherein the reaction conditions exemplified are heating 2,6-dichloro-4,8-dipiperidino- pyrimido(5,4-d)pyrimidine and diethanolamine at 155 to 160°C under vacuum. However, this process again requires a high temperature which leads to the formation of impurities.A process for the preparation and purification of dipyridamole is disclosed in patent DE 1812918, wherein 2,6-dicMoro-4,8-dipiperidino-pyrimido(5,4-d)pyrimidine and diethanolamine are heated to 150 to 200°C. After completion of the reaction, the reaction mixture is dissolved in chloroform, which is further separated into an upper layer of diethanolamine and its hydrochloride and a chloroform solution. The chloroform solution obtained is separated and reduced to dryness after stirring with water. This process also requires a high temperature which can lead to the formation of impurities. In addition, the solvent used for the isolation of dipyridamole, chloroform, is inconvenient as it is a restricted solvent and its permitted limit in the final marketed dipyridamole is very low.A similar process, wherein dipyridamole is manufactured by the reaction of diethanolamine with 2,6-dichloro-4,8-dipiperidino-pyrimido(5,4-d)pyrimidine is disclosed in patent RO 104718. However, this process again requires high temperatures of 180 to 200°C which leads to the formation of impurities and, consequently, the yield of the final product is very low (58%) with a purity of less than 98%.A process is disclosed in patent DD 115670, wherein the purification of dipyridamole involves refluxing it in butyl acetate, AcOBu, for 2 hours in the presence of an equal amount of silica gel or column chromatography on silica gel at 60-100°C. However, purification by column chromatography is not economical and not feasible on industrial scale. Moreover, this purification process only removes one specific impurity, 2,4,6-tris- (diethanolamino) – 8 -pip eridino-pyrimido (5,4-d)pyrimidine .The processes described above to prepare dipyridamole do not employ an additional reaction solvent but involve neat mixtures of the two reactants, 2,6-dichloro-4,8- dipiperidino-pyrimido(5,4-d)pyrimidine and diethanolamine, which are heated at very high temperatures. The use of neat reaction mixtures and/ or high temperatures means that it is very difficult to control the levels of impurities formed.Another process for the preparation of dipyridamole, disclosed in patent application WO 2007/080463, involves reacting diethanolamine with 2,6-dichloro-4,8-dipiperidino- pyrimido(5,4-d)pyrimidine in a solvent selected from the group consisting of l-methyl-2- pyrrolidinone, sulpholane and polyethylene glycol. However, the exemplified reaction temperatures are very high at 190 to 200°C and the HPLC purity of the crude dipyridamole is reported to be only 90-94%. A purification method is disclosed using first a ketonic solvent and then an alcohol and water. Even though the process disclosed in this patent application uses a solvent in the reaction, the temperature of reaction is still very high and the purification in ketonic solvent is reported at high temperature (100 to 120°C). The HPLC purity after purification is reported as only 99.0-99.5%.As discussed above, all the processes disclosed in the prior art for the preparation of dipyridamole suffer from serious disadvantages with respect to commercial production. The prior art synthetic and purification processes employ high temperatures in the preparation of dipyridamole which leads to inefficiency and high processing costs. The high temperatures also lead to higher levels of impurities being formed during manufacture with the consequence that further cumbersome and expensive purification procedures are required.The high quality dipyridamole prepared by the processes according to the present invention can be used for the preparation of a pharmaceutical composition to use in the manufacture of a medicament for anti-platelet therapy. A preferred embodiment of the present invention, illustrated in Scheme 2, provides a process for the preparation of dipyridamole comprising reacting 2,6-dichloro-4,8- dipiperidino-pyrimido(5,4-d)pyrimidine with diethanolamine at 113-115°C. This reaction temperature is significantly lower than that used in the prior art processes to prepare dipyridamole.

Another preferred embodiment of the present invention, illustrated in Scheme 3, also provides a process for the preparation of dipyridamole by the reaction of 2,6-dichloro-4,8- dipiperidino-pyrimido(5,4-d)pyrimidine with diethanolamine in dimethylsulfoxide at 120- 125°C to afford the mono-substituted intermediate, 2-chloro-6-diethanolamino-4,8- dipiperidino-pyrimido(5,4-d)pyrimidine, which is isolated and then further converted to dipyridamole by heating in diethanolamine at 113-115°C.Although the solvent used in this preferred embodiment of the present invention is preferably dimethylsulfoxide (DMSO), other solvents can alternatively be used. Preferred alternative solvents are other polar aprotic solvents, such as dimethylformamide (DMF), dimethylacetamide (DMA) or N-methyl-2-pyrrolidinone (NMP). Alternatively, hydrocarbon solvents can be used. Preferred hydrocarbon solvents are aromatic hydrocarbon solvents such as toluene or xylene.


Example 1Preparation of crude dipyridamoleDiethanolamine (10 vol) and 2,6-dichloro-4,8-dipiperidino-pyrimido(5,4-d)pyrimidine (1 eq) were mixed at 25-30°C, stirred for 10 minutes and then heated at 113-115°C for 45-48 hours. After completion of the reaction, the mixture was cooled to 75-80°C. Ethanol (5 vol) was added at 75-80°C and the mixture was stirred at 75-80°C for 10 minutes. Toluene (10 vol) was added at 70-75°C and the mixture was stirred at 70-75°C for 15 minutes. Purified water (15 vol) was added at 70-75°C and the mixture was stirred at 60-65°C for 30 minutes. The mixture was then cooled and stirred at 25-30°C for 30 minutes. The precipitated solid was filtered and washed with purified water (2 x 5 vol) before drying at 75-80°C under reduced pressure afforded crude dipyridamole as a yellow crystalline solid. Yield (w/w) = 80-85%Yield (molar) = 58-62%HPLC purity > 98%Example 2Stage 1: Preparation of 2-chloro-6-diemanolamino-4,8-dipiperidino-pyrimido(5,4-d) pyrimidineDiethanolamine (3 eq) and 2,6-dichloro-4,8-dipiperidino-pyrimido(5,4-d)pyrimidine (1 eq) were added to dimefhylsulfoxide (10 vol) at 25-30°C, stirred for 10 minutes and then heated at 120-125°C for 4-5 hours. After completion of the reaction, the reaction mixture was cooled to 55-60°C. Acetone (5 vol) was added at 55-60°C and the mixture was stirred at 55-60°C for 10 minutes. Purified water (15 vol) was added at 55-60°C and the mixture was stirred at 50-55°C for 15 minutes. The mixture was cooled to 25-30°C and stirred at 25-30°C for 30 minutes. The precipitated solid was filtered, washed with purified water (2 x 5 vol) and dried at 75-80°C under reduced pressure to afford crude 2-chloro-6- diethanolamino-4,8-dipiperidino-pyrimido(5,4-d)pyrimidine as a yellow crystalline solid. Yield (w/w) = 110-120%Yield (molar) = 93-100%HPLC purity > 96%Stage 2: Preparation of crude dipyridamoleDiethanolamine (10 vol) and 2-chloro-6-diemanolamino-4,8-dipiperidino-pyrimido(5,4-d) pyrimidine (1 eq) were mixed at 25-30°C, stirred for 10 minutes and then heated at 113- 115°C for 45-48 hours. After completion of the reaction, the mixture was cooled to 75- 80°C. Ethanol (5 vol) was added and the mixture was stirred at 75-80°C for 10 minutes. Toluene (10 vol) was added and the mixture was stirred at 70-75°C for 15 minutes. Purified water (15 vol) was added and the mixture was stirred at 60-65°C for 30 minutes. The mixture was then cooled to 25-30°C and stirred for 30 minutes. The precipitated solid was filtered, washed with purified water (2 x 5 vol) and dried at 75-80°C under reduced pressure to afford crude dipyridamole as a yellow crystalline solid.Yield (w/w) = 95-97%Yield (molar) = 82-84%HPLC purity > 98%Example 3Crystallization of crude dipyridamoleCrude dipyridamole (1 eq) and diefhanolamine (8 vol) were stirred together at 25-30°C for 10 minutes and then heated to about 80°C for 10 minutes. The clear solution was cooled to 75-80°C, ethanol (5 vol) was added and the mixture was stirred at 75-80°C for 10 minutes. Toluene (10 vol) was added and the mixture was stirred at 70-75°C for 15 minutes. The mixture was cooled to 25-30°C, stirred at 25-30°C for 10 minutes and filtered. The filtrate was heated to 70-75°C for 10 minutes, purified water (15 vol) was added and the mixture was stirred at 60-65°C for 30 minutes before cooling to 25-30°C with stirring for 30 minutes. The precipitated solid was filtered, washed with purified water (2 x 5 vol) and dried at 75-80°C under reduced pressure to afford dipyridamole as a yellow crystalline solid.Yield (w/w and molar) = 90-95%HPLC purity > 99.9%
PATENT
https://patents.google.com/patent/WO2007080463A1/enDipyridamole which is chemically known as 4,8-Bis(piperidino)-N,N,N’,N’- tetra(2-hydroxyethyl)pyrimido[5,4-d]pyrimidine-2,6-diamine is a platelet adhesion inhibitor. It is useful in anti-platelet therapy and it is marketed as Persantin ® by Boehringer Ingelheim.The platelet aggregation inhibiting properties, anti-thrombotic and vasodilator properties of Dipyridamole is reported in US patent 3031450 which also describe a process for its preparation by reacting 2-chloro-6-diethanolamino-4, 8-dipiperidyl- pyrimido-pyrimidine with diethanolamine.German patent 117456 describes the process for the production of Dipyridamole from 2,6-dichloro-4,8-dipiperidinopyrimido[5,4-d]pyrimidine and diethanolamine at 130 to 200° C under vacuum. German patent 1812918 describes the process for the preparation and purification of Dipyridamole. According to this patent 2,6-dichloro-4,8,- dipiperidinopyrimido[5,4-d]pyrimidine and diethanolamine are heated to 150 to 2000C to obtain Dipyridamole. This is characterized by the fact that after the completion of the reaction, the reaction mixture is dissolved in chloroform, which is further separated into the upper layer of diethanolamine and its hydrochloride and the chloroform solution. Thus obtained chloroform solution is reduced to dryness after stirring with water.RO 104718 Bl describes a process where Dipyridamole is manufactured and purified by reaction of diethanolamine with 2,6-dichloro-4,8-dipiperidinopyrimido[5,4- d]pyrimidine. In this process the yield is very low (58%) and purity is only 98%.Another patent DDl 15670 Z describes a process for the purification of Dipyridamole by refluxing it in AcOBu for 2 h in the presence of an equal amount of silica gel or by column chromatography on silica gel at 60-1000C. The purification by column chromatography is not economical and feasible at industrial scale.All the above mentioned prior art are neat reaction in which it is difficult to control the impurity and is not easy to scale up. In the prior art process the obtained product is pasty which needs decanting the mother liquor and further purification.We focused our research to develop an improved and efficient process for the preparation and purification of Dipyridamole of formula (I) which will overcome the above mentioned prior art problems and will produce Dipyridamole in substantially good yield, high purity and with no mixture of solvents.Objectives of the InventionThe main objective of the present invention is to provide an improved process for the preparation and purification of compound of formula (I), which gives better purity and high yield of the product. Another objective of the present invention is to provide a process for the preparation and purification of compound of formula (I), which would be easy to scale up and implement at industrial level.Yet another objective of the present invention is to provide a process for the preparation and purification of compound of formula (I), which avoids, use of hazardous gas (SO2) and corrosive chemicals like HCl, H2SO4, acetic acid, NaOH, NH3 etc.Summary of the InventionAccordingly, the present invention provides a process for the preparation ofDipyridamole of formula (I) comprising reacting 2,6-Dichloro-4,8- dipiperidinopyrimido- (5,4-d)pyrimidine (DDH) of formula (II) with Diethanolamine (DEA) of formula (III) using a solvent. This process can be represented by the scheme given below:
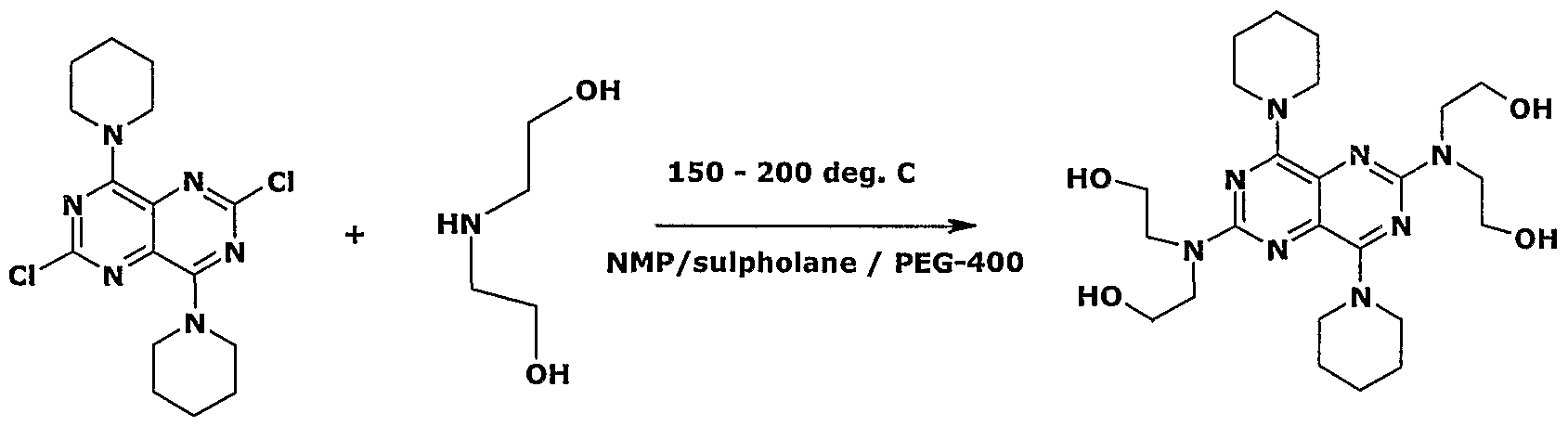
(II) (III) (I)The obtained wet or optionally dried crude Dipyridamole is purified by using ketonic solvent and aqueous alcoholic solvent or mixture thereof.Description of the InventionIn an embodiment of the present invention the solvent is selected from the group consisting of l-Methyl-2-pyrrolidinone, Sulpholane and Polyethylene glycol, preferably l-Methyl-2-pyrrolidinone (NMP). In another embodiment of the present invention, the polyethylene glycol used is PEG-20Q or PEG-400, preferably PEG-400.In yet another embodiment of the present invention, the reaction is carried out at a temperature of about 25° C to reflux temperature, preferably at a temperature of about 150° C to 2000C.In still another embodiment of the present invention the starting material of this invention is prepared according to the literature available in the prior art.In yet another embodiment the ketonic solvent is acetone, methyl ethyl ketone, methyl vinyl ketone or methyl isobutyl ketone (MIBK), preferably MIBK.In yet another embodiment the alcoholic solvent is selected from the group having Ci to C4 alkanol preferably isopropyl alcohol (IPA) or methanol.The present invention is illustrated with the following examples, which should not be construed for limiting the scope of the invention.Example 1Preparation of Dipyridamole (Crude)250 mL of l-Methyl-2-pyrrolidinone (NMP), 50g of 2,6-Dichloro-4,8 dipiperidinopyrimido(5,4-d)pyrimidine (DDH) and 136g of Diethanolamine (DEA) were charged into a 2.0 L four-necked RBF at 25-35 0C. The reaction mass was heated to 190 – 2000C and maintained for 1.5 to 2.5 hrs under stirring. The reaction mass was cooled to 25-350C and 450 mL of purified water was charged slowly into it and stirred for lhr. The solid reaction mass was filtered and washed with 500ml-purified water and dried the solid under vacuum at 50-550C for 8 to 10 hrs to get 55-60 g of crude Dipyridamole of 90-94% HPLC purity.Purification Of DipyridamoleMethyl isobutyl ketone (750 ml) and 50 g of Dipyridamole (crude) were charged into a clean 2.0 L four-necked RBF at 25-3O0C and heated to 100-1200C. It was stirred to dissolve and cooled to 25-350C and stirred for 30-60 min. The solid was Filtered and washed with 100 ml MIBK. The product was dried at 45-50 0C under vacuum. The obtained material was further purified as follows:Isopropyl alcohol (200 mL) and 40-45 g of Dipyridamole were charged into a clean 1.0 lit four-necked RBF at 25-350C. It was heated to 60-650C. Carbon (Ig) was added at 30-350C and filtered through celite and washed with 50 mL IPA. Water (500 mL) was charged slowly and stirred for 30 min. The solid was filtered and washed with a mixture of IPA : Water (1 :2) and dried the product at 45-50 0C under vacuum to obtain 43-5Og of Dipyridamole having HPLC purity 99.0 – 99.5%Example 2Sulpholane (15 mL), 5.0g of 2,6-Dichloro-4,8-dipiperidinopyrimido(5,4-d) pyrimidine (DDH) and 8.6g Diethanolamine (DEA) were charged into 100 mL four- necked RBF at 25-35°C. It was heated to 190-2000C and stirred for 2-3 hrs. The reaction mass was cooled to 25-35°C and 45 mL of water was added into it. The reaction mass was stirred. The solid was filtered and washed with water. The solid was purified with MIBK,IPA-Water as given in example 1.Example 3Polyethylene glycol-400 (15 mL), 5g of 2,6-Dichloro-4,8-dipiperidinopyrimido (5,4-d) pyrimidine (DDH) and 8.6 gm Diethanolamine (DEA) were charged into 10OmL four necked RBF at 25-35°C. The reaction mass was heated to 190-2000C and maintained for 2-3 hrs. The mixture was cooled to 25-350C. Water (45 mL) was added to the reaction mixture and stirred. The solid was filtered and washed with water. The solid was purified with MIBK,IPA- Water as given in example 1.Example 4 (Azeotrophic removal of water in MIBK purification)Preparation of Dipyridamole (Crude*) l-Methyl-2-pyrrolidinone (150 mL), 50g of 2,6-Dichloro-4,8-dipiperidinopyrimido(5,4-d)pyrimidine (DDH) and 136g Diethanolamine (DEA) was charged into a 2.0 L four-necked RBF at 25-35 0C. The reaction mass was stirred & heated to 190 – 2000C and stirring was continued for 1.5 to 2.5 hrs. The reaction mass was cooled to 25-350C and 450 mL of purified water was charged slowly into it and stirred for lhr. The solid reaction mass was filtered and washed with 500ml-purified water to obtain 110-13Og of wet crude Dipyridamole.Purification Of DipyridamoleMethyl isobutyl ketone (750 ml) and 65 g of Dipyridamole (wet crude) were charged into a clean 2.0 L four-necked RBF at 25-3O0C and heated to 100-1200C followed by azeotrophic separation of water. It was cooled to 25-350C and stirred for30-60 min. The solid was filtered and washed with 100 ml MIBK. The product was dried at 45-50 0C under vacuum. The obtained material was further purified as follows:Isopropyl alcohol (200 mL) and 45-48 g of Dipyridamole were charged into a clean 1.0 L four-necked RBF at 25-350C. It was heated to 60-650C and stirred to dissolve. Carbon (Ig) was added at 30-350C and filtered through celite and washed with 50 mL IPA. Water (500 mL) was charged slowly and stirred for 30 min. The solid was filtered and washed with a mixture of IPA : Water (1:2) and dried the product at 45-50 0C under vacuum to obtain 43-5Og of Dipyridamole having HPLC purity 99.0-99.5%Purification with Methaanol-waterMethanol (200 mL) and 45-48 g of Dipyridamole were charged into a clean 1.0 L four-necked RBF at 25-350C. It was heated to 60-650C and stirred to dissolve. Carbon (Ig) was added at 30-350C and filtered through celite and washed with 50 mL methanol. Water (500 mL) was charged slowly and stirred for 30 min. The solid was filtered and washed with a mixture of methanol : Water (1 :2) and dried the product at 45-50 0C under vacuum to obtain 45g of Dipyridamole having HPLC purity 99%.
PATENThttps://patents.google.com/patent/CN108069972A/enEmbodiment 1Weigh urea 120g(2mol), ethylene glycol 62g(1mol)120 DEG C are heated in a kettle, in Catalyzed by p-Toluenesulfonic Acid Effect is lower to carry out step(1)Reaction, generate compound 3;Then 2,3- diamino succinic acid 37g is weighed(0.25mol)With step The compound 3 generated in rapid 1 carries out the reaction generation compound 5 of step 2,220 DEG C, reaction time 3h of reaction temperature, catalyst For the nickel-base catalyst of support type, catalyst is filtered out after the completion of reaction;Exist in phosphorus oxychloride, phosphorus trichloride and lead to chlorine In the case of, compound 5 carries out chlorination reaction generation compound 6,110 DEG C of reaction temperature, reaction time 30h;Weigh piperidines 85g (1mol)Step is carried out with compound(4)Reaction, after reaction liquid hydrolysis, cooling filtering, be dried to obtain compound 9;Claim Take 105g(1mol)Diethanol amine and compound 9 carry out step(6)Reaction, 220 DEG C of reaction temperature, reaction time 3h fills 1.5 MPa of Hydrogen Vapor Pressure, catalyst are the crude product of the nickel-base catalyst, after reaction cold filtration of support type, and crude product passes through It is refining to obtain Dipyridamole finished product, quality 62.10g, purity 99.21%, product yield 48.74%(Yield is with 2,3- diamino fourths Diacid is calculating benchmark).Embodiment 2Weigh urea 120g(2mol), ethylene glycol 62g(1mol)120 DEG C are heated in a kettle, in Catalyzed by p-Toluenesulfonic Acid Effect is lower to carry out step(1)Reaction, generate compound 3;Then 2,3- diamino succinic acid 30g is weighed(0.2mol)With step The compound 3 generated in rapid 1 carries out the reaction generation compound 5 of step 2,220 DEG C, reaction time 3h of reaction temperature, catalyst For the nickel-base catalyst of support type, catalyst is filtered out after the completion of reaction;Exist in phosphorus oxychloride, phosphorus trichloride and lead to chlorine In the case of, compound 5 carries out chlorination reaction generation compound 6,110 DEG C of reaction temperature, reaction time 30h;Weigh piperidines 85g (1mol)Step is carried out with compound(4)Reaction, after reaction liquid hydrolysis, cooling filtering, be dried to obtain compound 9;Claim Take 105g(1mol)Diethanol amine and compound 9 carry out step(6)Reaction, 220 DEG C of reaction temperature, reaction time 3h fills 1.5 MPa of Hydrogen Vapor Pressure, catalyst are the crude product of the nickel-base catalyst, after reaction cold filtration of support type, and crude product passes through It is refining to obtain Dipyridamole finished product, quality 50.34g, purity 99.3%, product yield 49.53%(Yield is with 2,3- diamino fourth two Acid is calculating benchmark).Embodiment 3Other steps are the same as embodiment 2, step(6)In reaction temperature for 240 DEG C, product yield 50.31%.Comparative example 1Weigh urea 36g(0.6mol), ethyl acetoacetate 26g(0.2mol), add in ethanol-hydrogen chloride liquid(30% hydrochloric acid:95% second Alcohol=1:4)Then 200ml, drying and dehydrating after stirring add in sodium hydroxide solution and are warming up to 95 DEG C, then cool to 75 DEG C, add Hydrochloric acid adjusts PH=1, cold filtration, the 6- methyluracils of washing filtering;Nitric acid is added in reaction pot, is cooled to less than 10 DEG C, Stirring adds in 6- methyluracils, be warming up to 30 DEG C of heat preservations 1 it is small when the nitro whey liquid that filters;Take a policy powder in water, stirring After dissolving plus nitro whey liquid, temperature control keep the temperature 30min at 35 DEG C, add the static 3h of hydrochloric acid, stir 2h, filtration drying obtains amino breast Clear liquid;Again weigh urea 36g and add in reaction kettle with amino whey liquid, stirring is warming up to 100 DEG C of heat preservation 20min, cools to 90 DEG C add in 2mol/L sodium hydroxide solution, be warming up to 100 DEG C heat preservation dissolving 1h.Cool to 40 DEG C, filter tetrahydroxy pyrimidine- [4,5d] and pyrimidine sodium salt, adds water, 60 DEG C of heat preservation 30min add hydrochloric acid to adjust PH=4, is cooled to 15 DEG C of filterings, washing, dries 2,4,6,8- tetrahydroxys pyrimidine-[4,5d] and pyrimidine;Tetrahydroxy object, phosphorus oxychloride, phosphorus trichloride are added in reaction kettle, is stirred, 10 DEG C of similarly hereinafter chlorine are warming up to 110 DEG C of reflux for 24 hours, be cooled to 15 DEG C of filterings, washing, dry 2,4,6,8- tetrachloro-pyrimidines- [4,5d] and pyrimidine;Acetone, tetrachloride are sequentially added, piperidines-acetone mixture, 30 DEG C of heat preservations are added dropwise in 20 DEG C of heat preservation 30min 1h, adds water to stir 1h, and filtration drying obtains 2,6- bis- chloro- 4,8 ,-two piperidines-pyrimidine(Dichloride);Weigh diethanol amine 63g with Dichloride is mixed, and is warming up to 200 DEG C of heat preservation 15min, is cooled to less than 25 DEG C plus acetone and stirs 30min, then 30 DEG C 4h is kept the temperature, filtration drying obtains Dipyridamole crude product, then carries out refined Dipyridamole finished product 13.53, purity 98.5%, yield 13.21%(Using ethyl acetoacetate as calculating standard).It is found by being compared with comparative example:The method of the production Dipyridamole of the present invention is short with synthetic route, into The characteristics of product high income, production cost is low.
SYN
GB 807826 U.S. Patent 3,031,450

SYN
SYN
R.S. Vardanyan, V.J. Hruby, in Synthesis of Essential Drugs, 2006
Dipyridamole
Dipyridamole, 2,2′,2″,2′″-[(4,8-dipiperidinopirimido[5,4-d]pirimidin-2,6-diyl)-diimino]-tetraethanol (19.4.13), is easily synthesized from 5-nitroorotic acid (19.4.8), easily obtained, in turn, by nitrating of 2,4-dihydroxy-6-methylpyrimidine, which is usually synthesized by the condensation of urea with acetoacetic ether. Reduction of the nitro group in 5-nitroorotic acid by various reducing agents gives 5-aminoorotic acid (19.4.9), which is reacted with urea or with potassium cyanide to give 2,4,6,8-tetrahydroxypyrimido[5,4-d]pyrimidine (19.4.10). This undergoes a reaction with a mixture of phosphorous oxychloride and phosphorous pentachloride, which forms 2,4,6,8- tetra-chloropyrimido[5,4-d]pyrimidine (19.4.11). Reacting the resulting tetrachloride with piperidine replaces the chlorine atoms at C4 and C8 of the heterocyclic system with piperidine, giving 2,6-dichloropyrimido-4,8-dipiperidino[5,4-d]pyrimidine (19.4.12). Reacting the resulting product with diethanolamine gives dipyridamole (19.4.13) [32,33].

Dipyridamole increases coronary blood circulation, increases oxygen flow to the myocardium, potentiates adenosine activity, and impedes its metabolization. It inhibits aggregation of thrombocytes, blocks phosphodiesterase, increases microcirculation, and inhibits the formation of thrombocytes.
It is used for chronic coronary insufficiency, as well as for preventing and treating thrombosis. Synonyms of this drug are anginal, curantyl, stenocor, thrompresantin, and many others.SYN
Chemical Synthesis
Dipyridamole, 2,2′,2”,2”’-[(4,8-dipiperidinopirimido[5,4-d]pirimidin-2,6- diyl)-diimino]-tetraethanol (19.4.13), is easily synthesized from 5-nitroorotic acid (19.4.8), easily obtained, in turn, by nitrating of 2,4-dihydroxy-6-methylpyrimidine, which is usually synthesized by the condensation of urea with acetoacetic ether. Reduction of the nitro group in 5-nitroorotic acid by various reducing agents gives 5-aminoorotic acid (19.4.9), which is reacted with urea or with potassium cyanide to give 2,4,6,8- tetrahydroxypyrimido[5,4-d]pyrimidine (19.4.10). This undergoes a reaction with a mixture of phosphorous oxychloride and phosphorous pentachloride, which forms 2,4,6,8- tetrachloropyrimido[ 5,4-d]pyrimidine (19.4.11). Reacting the resulting tetrachloride with piperidine replaces the chlorine atoms at C4 and C8 of the heterocyclic system with piperidine, giving 2,6-dichloropyrimido-4,8-dipiperidino[5,4-d]pyrimidine (19.4.12). Reacting the resulting product with diethanolamine gives dipyridamole (19.4.13).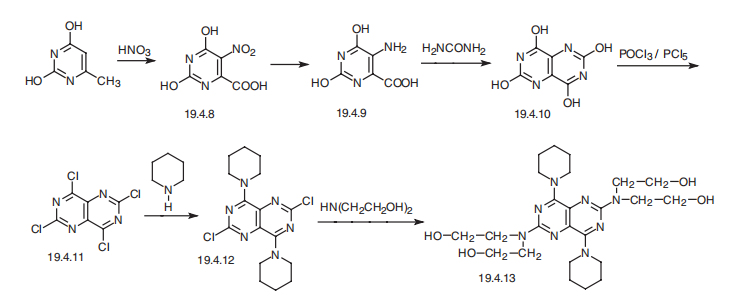
clipA general outline of the procedure for synthesizing dipyridamole is shown in Scheme 1. Reaction of the pyrimidino pyrimidine-2,4,6,8- tetraol (1) with a mixture of phosphorous oxychloride and phosphorous pentachloride gives the tetrachloro derivative (2). The halogens at the peri positions 4 and 8 are more reactive to substitution than are the remaining halogen pairs 2 and 6, which are in effect the two positions of the pyrimidines. Thus, reaction with piperidine at ambient temperature gives the 4, 8 diamine (3). Subsequent reaction with bis-2-hydroxy ethylamine under more strenuous conditions gives dipyridamole (4) [12, 13].12. F.G. Fischer, J. Roch, and A. Kottler, U.S. Patent 3, 031, 450 (1962). 13. D. Lednicer and L.A. Mitscher, The Organic Chemistry of Drug Synthessis Volume 1, John Wiley and Sons, New York, p. 428 (1977).
set 2
set 3

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Medical uses
- Dipyridamole is used to dilate blood vessels in people with peripheral arterial disease and coronary artery disease[4]
- Dipyridamole has been shown to lower pulmonary hypertension without significant drop of systemic blood pressure
- It inhibits formation of pro-inflammatory cytokines (MCP-1, MMP-9) in vitro and results in reduction of hsCRP[clarification needed] in patients.
- It inhibits proliferation of smooth muscle cells in vivo and modestly increases unassisted patency of synthetic arteriovenous hemodialysis grafts.[5]
- It increases the release of tissue plasminogen activator from brain microvascular endothelial cells.
- It results in an increase of 13-hydroxyoctadecadienoic acid and decrease of 12-hydroxyeicosatetraenoic acid in the subendothelial matrix and reduced thrombogenicity of the subendothelial matrix.
- Pretreatment it reduced reperfusion injury in volunteers.
- It has been shown to increase myocardial perfusion and left ventricular function in patients with ischemic cardiomyopathy.
- It results in a reduction of the number of thrombin and PECAM-1 receptors on platelets in stroke patients.
- Cyclic adenosine monophosphate impairs platelet aggregation and also causes arteriolar smooth muscle relaxation. Chronic therapy did not show significant drop of systemic blood pressure.
- It inhibits the replication of mengovirus RNA.[6]
- It can be used for myocardial stress testing as an alternative to exercise-induced stress methods such as treadmills.
Stroke
A combination of dipyridamole and aspirin (acetylsalicylic acid/dipyridamole) is FDA-approved for the secondary prevention of stroke and has a bleeding risk equal to that of aspirin use alone.[4] Dipyridamole absorption is pH-dependent and concomitant treatment with gastric acid suppressors (such as a proton pump inhibitor) will inhibit the absorption of liquid and plain tablets.[7][8] Modified release preparations are buffered and absorption is not affected.[9][10]
However, it is not licensed as monotherapy for stroke prophylaxis, although a Cochrane review suggested that dipyridamole may reduce the risk of further vascular events in patients presenting after cerebral ischemia.[11]
A triple therapy of aspirin, clopidogrel, and dipyridamole has been investigated, but this combination led to an increase in adverse bleeding events.[12]
- Vasodilation occurs in healthy arteries, whereas stenosed arteries remain narrowed. This creates a “steal” phenomenon where the coronary blood supply will increase to the dilated healthy vessels compared to the stenosed arteries which can then be detected by clinical symptoms of chest pain, electrocardiogram and echocardiography when it causes ischemia.
- Flow heterogeneity (a necessary precursor to ischemia) can be detected with gamma cameras and SPECT using nuclear imaging agents such as Thallium-201, Tc99m–Tetrofosmin and Tc99m–Sestamibi. However, relative differences in perfusion do not necessarily imply any absolute decrease in blood supply in the tissue supplied by a stenosed artery.
Other uses
Dipyridamole also has non-medicinal uses in a laboratory context, such as the inhibition of cardiovirus growth in cell culture.[citation needed]
Drug interactions
Due to its action as a phosphodiesterase inhibitor, dipyridamole is likely to potentiate the effects of adenosine. This occurs by blocking the nucleoside transporter (ENT1) through which adenosine enters erythrocyte and endothelial cells.[13]
According to Association of Anaesthetists of Great Britain and Ireland 2016 guidelines, dipyridamole is considered to not cause risk of bleeding when receiving neuroaxial anaesthesia and deep nerve blocks. It does not therefore require cessation prior to anaesthesia with these techniques, and can continue to be taken with nerve block catheters in place.[14]
Overdose
Dipyridamole overdose can be treated with aminophylline[2]: 6 or caffeine which reverses its dilating effect on the blood vessels. Symptomatic treatment is recommended, possibly including a vasopressor drug. Gastric lavage should be considered. Since dipyridamole is highly protein bound, dialysis is not likely to be of benefit.
Mechanisms of action
Dipyridamole has two known effects, acting via different mechanisms of action:
- Dipyridamole inhibits the phosphodiesterase enzymes that normally break down cAMP (increasing cellular cAMP levels and blocking the platelet aggregation, response[4] to ADP) and/or cGMP.
- Dipyridamole inhibits the cellular reuptake of adenosine into platelets, red blood cells, and endothelial cells, leading to increased extracellular concentrations of adenosine.
Experimental studies[
Dipyridamole is currently undergoing repurposing for treatment of ocular surface disorders. These include pterygium and dry eye disease. The first report of topical dipyridamole’s benefit in treating pterygium was published in 2014.[15] A subsequent report of outcomes in 25 patients using topical dipyridamole was presented in 2016.[16]
See also
References
- ^ Nielsen-Kudsk, F; Pedersen, AK (May 1979). “Pharmacokinetics of Dipyridamole”. Acta Pharmacologica et Toxicologica. 44 (5): 391–9. doi:10.1111/j.1600-0773.1979.tb02350.x. PMID 474151.
- ^ Jump up to:a b “Aggrenox (aspirin/extended-release dipyridamole) Capsules. Full Prescribing Information” (PDF). Boehringer Ingelheim Pharmaceuticals, Inc. Retrieved 1 December 2016.
- ^ “Dipyridamole” at Dorland’s Medical Dictionary
- ^ Jump up to:a b c Brown DG, Wilkerson EC, Love WE (March 2015). “A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons”. Journal of the American Academy of Dermatology. 72 (3): 524–34. doi:10.1016/j.jaad.2014.10.027. PMID 25486915.
- ^ Dixon BS, Beck GJ, Vazquez MA, et al. (2009). “Effect of dipyridamole plus aspirin on hemodialysis graft patency”. N Engl J Med. 360 (21): 2191–2201. doi:10.1056/nejmoa0805840. PMC 3929400. PMID 19458364.
- ^ Dipyridamole in the laboratory: Fata-Hartley, Cori L.; Ann C. Palmenberg (2005). “Dipyridamole reversibly inhibits mengovirus RNA replication”. Journal of Virology. 79 (17): 11062–11070. doi:10.1128/JVI.79.17.11062-11070.2005. PMC 1193570. PMID 16103157.
- ^ Russell TL, Berardi RR, Barnett JL, O’Sullivan TL, Wagner JG, Dressman JB. pH-related changes in the absorption of “dipyridamole” in the elderly. Pharm Res (1994) 11 136–43.
- ^ Derendorf H, VanderMaelen CP, Brickl R-S, MacGregor TR, Eisert W. “Dipyridamole” bioavailability in subjects with reduced gastric acidity. J Clin Pharmacol (2005) 45, 845–50.
- ^ “Archived copy”. Archived from the original on 2009-07-05. Retrieved 2010-02-06.
- ^ Stockley, Ivan (2009). Stockley’s Drug Interactions. The Pharmaceutical Press. ISBN 978-0-85369-424-3.
- ^ De Schryver EL, Algra A, van Gijn J (2007). Algra A (ed.). “Dipyridamole for preventing stroke and other vascular events in patients with vascular disease”. Cochrane Database of Systematic Reviews (2): CD001820. doi:10.1002/14651858.CD001820.pub3. PMID 17636684.
- ^ Sprigg N, Gray LJ, England T, et al. (2008). Berger JS (ed.). “A randomised controlled trial of triple antiplatelet therapy (aspirin, clopidogrel and dipyridamole) in the secondary prevention of stroke: safety, tolerability and feasibility”. PLOS ONE. 3 (8): e2852. Bibcode:2008PLoSO…3.2852S. doi:10.1371/journal.pone.0002852. PMC 2481397. PMID 18682741.
- ^ Gamboa A, Abraham R, Diedrich A, Shibao C, Paranjape SY, Farley G, et al. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005;36(10):2170-2175.
- ^ AAGBI Guidelines Neuraxial and Coagulation June 2016
- ^ Carlock, Beth H.; Bienstock, Carol A.; Rogosnitzky, Moshe (2014-03-25). “Pterygium: Nonsurgical Treatment Using Topical Dipyridamole – A Case Report”. Case Reports in Ophthalmology. 5 (1): 98–103. doi:10.1159/000362113. ISSN 1663-2699. PMC 3995373. PMID 24761148.
- ^ “Topical Dipyridamole for Treatment of Pterygium and Associated Dry Eye Symptoms: Analysis of User-Reported Outcomes”. ResearchGate. Retrieved 2019-05-19.
| Clinical data | |
|---|---|
| Trade names | Persantine, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682830 |
| Pregnancy category | B |
| Routes of administration | By mouth, IV |
| ATC code | B01AC07 (WHO) |
| Legal status | |
| Legal status | UK: POM (Prescription only)US: ℞-only |
| Pharmacokinetic data | |
| Bioavailability | 37–66%[1] |
| Protein binding | ~99% |
| Metabolism | Liver (glucuronidation)[2] |
| Elimination half-life | α phase: 40 min, β phase: 10 hours |
| Excretion | Biliary (95%), urine (negligible) |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 58-32-2 |
| PubChem CID | 3108 |
| IUPHAR/BPS | 4807 |
| DrugBank | DB00975 |
| ChemSpider | 2997 |
| UNII | 64ALC7F90C |
| KEGG | D00302 |
| ChEBI | CHEBI:4653 |
| ChEMBL | ChEMBL932 |
| CompTox Dashboard (EPA) | DTXSID6040668 |
| ECHA InfoCard | 100.000.340 |
| Chemical and physical data | |
| Formula | C24H40N8O4 |
| Molar mass | 504.636 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| hideSMILESOCCN(CCO)C(N=C1N2CCCCC2)=NC3=C1N=C(N(CCO)CCO)N=C3N4CCCCC4 | |
| showInChI | |
| (verify) |
Patent
Publication numberPriority datePublication dateAssigneeTitleUS3031450A1959-04-301962-04-24Thomae Gmbh Dr KSubstituted pyrimido-[5, 4-d]-pyrimidinesDE1812918A11968-04-251969-11-06Dresden Arzneimittel2,6-Bis (diethanolamino-4,8-dipiperidino-pyrimido (5,4-d)-pyrimidine – purification by simple procedure giving good yieldsDD115670A11974-02-191975-10-12DD117456A11975-02-131976-01-12DE2927539A1 *1979-07-071981-01-08Margineanu Dan Axente Dipl IngBis:di:ethanol-amino-di:piperidino-pyrimido-pyrimidine prepn. – from methyl acetoacetate and urea via amino-orotic acidRO104718B11989-08-091994-09-30Medicamente DePRODUCTION METHOD OF PURE 2,6-bis-(DIETHANOL AMIDE)-4,8-DI- PIPERIDINE-PYRIMIDO-(5,4-d)-PYRIMIDINEWO2007080463A12006-01-122007-07-19Orchid Chemicals & Pharmaceuticals LimitedAn improved process for the preparation of dipyridamoleFamily To Family CitationsDE115670C *JPS5191295A *1975-02-051976-08-10Jipiridamooruno kairyoseizohoJPS5757038B2 *1977-09-301982-12-02Yamanouchi Pharma Co LtdJPS57209291A *1981-06-171982-12-22Kyowa Hakko Kogyo Co LtdPurification of dipyridamoleUS6232312B1 *1995-06-072001-05-15Cell Pathways, Inc.Method for treating patient having precancerous lesions with a combination of pyrimidopyrimidine derivatives and esters and amides of substituted indenyl acetic acidesCN1425461A *2003-01-032003-06-25贵州益佰制药股份有限公司Injection preparation for resisting platelet aggregation and its producing methodCN1634085A *2004-11-242005-07-06崔晓廷Injectio of aspirin and dipyridamole and its preparing process
Non-Patent
TitleCURTIN, NICOLA J. ET AL: “Resistance-Modifying Agents of Pyrimido[5,4-d]pyrimidine Modulators of Antitumor Drug Activity. Synthesis and Structure-Activity Relationships for Nucleoside Transport Inhibition and Binding to .alpha.1-Acid Glycoprotein”, JOURNAL OF MEDICINAL CHEMISTRY , 47(20), 4905-4922 CODEN: JMCMAR; ISSN: 0022-2623, 26 August 2004 (2004-08-26), XP002651697 *
CN104710431B *2015-03-182017-03-01常州康普药业有限公司A kind of purifying process of dipyridamoleCN107782805B *2016-08-252021-02-02亚宝药业集团股份有限公司HPLC analysis method for key intermediate impurity synthesized by dipyridamoleCN106380471B *2016-08-312018-11-06广州市桐晖药业有限公司A kind of preparation method of DipyridamoleCN108069972A *2016-11-162018-05-25湖南尔康制药股份有限公司A kind of production method of Dipyridamole bulk pharmaceutical chemicalsCN106946887B *2017-03-242019-05-28大连万福制药有限公司A kind of preparation method introducing catalyst optimization synthesis Dipyridamole
/////////////////Dipyridamole, дипиридамол , ديبيريدامول , 双嘧达莫 , 0068373 , NSC-515776, RA-8
OCCN(CCO)C(N=C1N2CCCCC2)=NC3=C1N=C(N(CCO)CCO)N=C3N4CCCCC4
