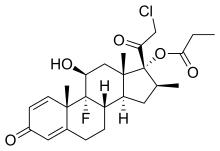
Clobetasol propionate
- Molecular FormulaC25H32ClFO5
- Average mass466.970 Da
CCI 4725, CCI-4725, GR 2/925, GR-2/925,(8S,9R,10S,11S,13S,14S,16S,17R)-17-(chloroacetyl)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl propanoate
246-634-3[EINECS], 25122-46-7[RN]
(11β,16β)-21-chloro-9-fluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl propanoate
Active Moieties
| NAME | KIND | UNII | CAS | INCHI KEY |
|---|---|---|---|---|
| Clobetasol | prodrug | ADN79D536H | 25122-41-2 | FCSHDIVRCWTZOX-DVTGEIKXSA-N |
Clobetasol
CAS Registry Number: 25122-41-2
CAS Name: (11b,16b)-21-Chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione
Molecular Formula: C22H28ClFO4, Molecular Weight: 410.91
Percent Composition: C 64.30%, H 6.87%, Cl 8.63%, F 4.62%, O 15.57%
Literature References: Topical corticosteroid. Prepn: Elks et al.,DE1902340; eidem,US3721687 (1969, 1973 both to Glaxo). Review of pharmacology and clinical efficacy in skin disorders: E. A. Olsen, R. C. Cornell, J. Am. Acad. Dermatol.15, 246-255 (1986).
Derivative Type: 17-Propionate
CAS Registry Number: 25122-46-7
Manufacturers’ Codes: GR-2/925
Trademarks: Clobesol (GSK); Dermovate (GSK); Olux (Connetics); Psorex (GSK); Temovate (GSK)
Molecular Formula: C25H32ClFO5
Molecular Weight: 466.97
Percent Composition: C 64.30%, H 6.91%, Cl 7.59%, F 4.07%, O 17.13%
Properties: White or almost white colorless, crystalline powder, mp 195.5-197°. [a]D +103.8° (c = 1.04 in dioxane). uv max (ethanol): 237 nm (e 15000). Insol in water.
Melting point: mp 195.5-197°
Optical Rotation: [a]D +103.8° (c = 1.04 in dioxane)
Absorption maximum: uv max (ethanol): 237 nm (e 15000)
Therap-Cat: Glucocorticoid; anti-inflammatory.
Keywords: Glucocorticoid.
Clobetasol propionate is a corticosteroid used to treat corticosteroid-responsive dermatoses and plaque psoriasis.
Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, and psoriasis.[2] It is applied to the skin as a cream, ointment, or shampoo.[2][3] Use should be short term and only if other weaker corticosteroids are not effective.[3] Use is not recommended in rosacea or perioral dermatitis.[2]
Common side effects include skin irritation, dry skin, redness, pimples, and telangiectasia.[2] Serious side effects may include adrenal suppression, allergic reactions, cellulitis, and Cushing’s syndrome.[2] Use in pregnancy and breastfeeding is of unclear safety.[4] Clobetasol is believed to work by activating steroid receptors.[2] It is a US Class I (Europe: class IV) corticosteroid, making it one of the strongest available.
Clobetasol propionate was patented in 1968 and came into medical use in 1978.[5] It is available as a generic medication.[3] In 2019, it was the 180th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[6][7]
SYNTHESIS OF KEY INTERMEDIATE
SYN

DE 1902340
US 3992422
DE 2613875
EP 72200
WO 2012122452
CN 112110972
PATENT
IN 201821008147
Clobetasol propionate (C25H32ClFO5); CAS Registry No.[25112-46-7]; IUPAC name: 17-(2′- Chloroacetyl)-9-fluoro-l l-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,l 1,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propionate is a potent halogen adrenal corticosteroid of the gluco-corticoid class used to treat various skin disorders including eczema and psoriasis. It is also highly effective for contact dermatitis caused by exposure to poison ivy/oak.In the US 3721687Apatentshow use of methanesulfonyl chloride and Pyridine as base to protect alcohol and at the time of LiCI reaction results with 10-15%ene impurity and less yield.In the methanesulfonyl chloride step used with pyridine as base which is a hazardous.Mesyl compound converted to Clobetasol propionate by using LiCl in Dimethylformamide reaction at IOO-IlO0C forms 10-15% with ene impurity. The synthesis of Clobetasol propionate results in small quantities of the eneimpurity. Clobetasol propionate desired compound to be with impurities which must be minimized. Ene impurity can be reduced to very low levels by reaction itself. However, if used recrystallization reduce ene impurity it is time consuming and very expensive. Further, because recrystallizations have high losses, unacceptably low yields.


Example I: Betamethasone to betamethasone 17- propionate To a 100 ml 4-neck round bottom flask (RBF) equipped with halfmoon stirrer, thermowelland addition funnel, mounted in a tub bath, was charged betamethasone (5.0g, 0.0127mole), Dimethylformamide (20ml). Cooled the reaction mass to 10-15°C. Slowly added trimethyl ortho propionate (3.42g, 0.0255mole) and p-toluenesulfonic acid (PTSA)(0.30g, 0.00174 mole) to the reaction mass at 10-15°C. Stirred the contents 10-15°C for 4 hr. The reaction was monitored for completion by TLC. Further continued stirring at the same temperature for Ihr till reaction complies by TLC. After reaction completion, added H2SO4UP to pH=1.0-2.0 in to reaction mass.Reaction mass was quenched in Purified water (25ml) at 25-30°C. Cooled reaction mass temperature to 0-5°C. Stirred for I hr and filtered and washed with Purified water (10mlX2). Suck dried under vacuum completely to get cream coloured solid. Dried in tray drier at 50-55°C.Dry weight-5.40g(94.50%); HPLC: 98.5%;mp215-218°C. IR (KBr, on’):3454.90, 3370.99 (-OH); 1719.86, 1659.10 (C=O)iC25H33FO6;
MS 448.52m/z 449.2255 [M+H]; 1HNMR (300MHz, CDCl3S ppm): Spectrum is recorded on Varian, and Tetra Methyl Silane (TMS) as internal standard. 1H-NMR Spectrum shows Aromatic-HK-7.17-7.22(d,lH); Hj-6.37-6.38 (d,lH);Hr6.14 (s,lH); HF-4.04-4.06(s,2H);HE2.23-2.28 (q,2H);Hc-l.39-1.43 (d,3H); HB-1.14 (t,3H);HA-0.96-2.96 (m,20H). 13CMR (300MHz, CDCl3Sppm): 8.692 (CH2-CH3); 16.693; 19.664; 21.353; 23.042; 27.568; 30.387; 36.456; 43.400; 46.547; 47.422; 71.760; 93.547; 124.307; 125.728; 127.903; 129.222; 130.443; 132.472; 145.632; 153.044; 167.424; 175.031 (O-C=O); 185.772 (Cyclic C=O); 196.732 (CH-CO-CH2-OH).
Example 2: Betamethasone 17- propionateto betamethasone 21-tosylate 100ml 4-neck RBF equipped with halfmoon stirrer, thermowell, reflux condenser mountained in water bath, was charged Stage-1 (5.0g, O.Olllmole), Dimethylformamide (20ml). Added 4-Dimethylaminopyridine as base (4.10g, 0.0335mole) and p-toluenesulfonyl chloride (4.24.Og, 0.0222mole)slowly, Stirredfor2-3 hr at 25-30°C. Stirred reaction mass at 25-30°C till reaction complies by TLC.As such reaction mass used insitue for next step. Reaction mass aliquot taken (2ml) and quenched in DM water (20ml), precipited material fdtered and washed with DM water (20ml). Suck dried well. Dried in tray drier at 50-55°C to get dry white solid. Dry weight-0.598g, (89.0%); HPLC: 98.5%; mp-170-175°C (dec). IR (KBr,cm”1):3291.91, 2980.39 (-OH); 1739.15, 1661.99 (C=O); C32H39FO8S; MS 602.71mA 603.2317 [M+H]; 1HNMR (300MHz, CDCl3Sppm): Spectrum is recorded on Varian, and Tetra Methyl Silane (TMS) as internal standard. 1H-NMR Spectrum shows Aromatic-Ηκ7.17-7.22(d,lH); Hj-6.37-6.38 (d,lH);Hr6.14 (s,lH); HG-4.334-4.393 (m,lH);HF-3.846- 4.007(d,2H);HE-2.273-2.349 (q,2H);HD-l.671-1.688 (s,lH); Hc-I-306-1.331 (d,3H); Hb1.055-1.105 (t,3H);HA-0.941 -2.634 (m,18H).13CMR (300MHz, CDCl3Sppm): 9.055 (CH2- CH3); 17.244; 20.002; 21.353; 23.168; 27.901; 30.622; 33.508; 34.881; 36.783; 43.642; 46.637; 47.330; 48.113; 48.417; 66.613; 70.902; 93.801; 102.732; 124.415; 129.324; 130.443; 132.472; 145.632; 153.583; 168.042; 174.853 (O-C=O); 186.208 (Cyclic C=O); 205.491 (CH-CO-CH2-OAr).
Example 3:Betamethasone 21-tosylateto Clobetasol propionate As such reaction mass used insitue for next step. Added. lithium chloride (LiCl)1.04 gm (0.0245mole). Stirred the reaction mass at 60-65°C for 5-6 hr.Reaction completion checked by TLC.After reaction completion, Added DM water (200ml). Stirred the reaction mass at 10-15°C for Ihr and Filtered washed with DM water (30mlx2).Dried in oven at 50-55°C to get white crystalline powder. Dry weight-4.42gm, (85.0%); HPLC:99.70%;mp-158-161°C. IR (KBr, cm_1):3299.62, 2976.53 (-OH); 1734.32, (C=0);1662.95 (C=C);C25H32C1F05; MSΑβ6.9Ίτη/ζ 467 [M+H];’HNMR (300MHz, CDCl35ppm): Spectrum is recorded on Varian, and Tetra Methyl Silane (TMS) as internal standard. 1H-NMR Spectrum shows AromaticHk-7.094-7. 128(d,IH); Hj-6.267-6.307 (d,lH); Hr6.066-6.076 (s,IH); H0-4.334-4.393 (m,lH); Hf-3.846-4.007 (d,2H); HE-2.273-2.349 (q,2H); Hd-I .671-1.688 (s,lH); Hc-1.306- 1.331 (d,3H); Hb-I .055-1.105 (t,3H); HA-0.941-2.634 (m,17H).13CMR (300MHz, CDCl35ppm): 8.692 (CH2-CH3); 16.693; 19.664; 21.353; 23.042; 27.568; 30.387; 36.456; 41.104; 46.547; 47.422; 71.760; 93.547; 124.307; 125.728; 127.903; 129.222; 130.443; 132.472; 145.632; 153.044; 168.312; 173.101 (O-C=O); 185.802 (Cyclic C=O); 204.602(CH-CO-CH2-C1)
SYN
Ruben Vardanyan, Victor Hruby, in Synthesis of Best-Seller Drugs, 2016
Synthesis of clobetasol propionate (27.1.13) starts from the known betamethasone 17-propionate (27.1.26), a potent glucocorticoid steroid with antiinflammatory and immunosuppressive properties, which was mesylated with methanesulfonyl chloride in pyridine to produce 9α-fluoro-11β-hydroxy-21-methylsulfonyloxy-16β-methyl 17-propionyloxypregna-1,4-diene-3,20-dione (27.1.27). The obtained product was refluxed in acetone, DMF, and dry LiCl mixture to produce the desired clobetasol propionate (27.1.13) [34] (Scheme 27.2.).

Clobetasol propionate, its structural formula (formula (I)), is a potent halogen-containing adrenocorticoid drug, has strong anti-inflammatory, anti-pruritic and vasoconstrictive effects, and its anti-inflammatory effect is approximately hydrogenated It is 112 times that of cortisone, and it is also used to treat neurodermatitis, contact dermatitis, eczema, discoid lupus erythematosus and other symptoms. It is currently widely used in clinical practice. It has been very popular in the international market and ranks among the top hormones. At present, there are only a few domestic companies in normal production, and the total yield is about 88%.[0003]

[0004] Formula (I).[0005] The process route for the production of synthetic clobetasol propionate is complex, technically difficult, and product quality requirements are strict. This is due to the complex structure of corticosteroids. The chemical structure of this type of drug is composed of three six-membered rings and one five-membered ring fused together to form a special molecular structure composed of 21 carbon atoms, with special molecular configuration steric effects and steric barriers. Group role. The functional groups on the drug structure interfere with each other, which makes the chemical reaction very complicated. It is manifested in many synthetic process steps, low raw material utilization rate, large amount of auxiliary materials, long production cycle, and many side reactions. The reaction process has various problems such as a large amount of solvents, a large amount of waste water and waste gas, and difficulty in recycling. Low technical indicators, low cost and other aspects.[0006] US patent, patent number 3721687, discloses two synthetic processes.[0007] Process method (1) adopts 9a-fluoro-113-hydroxy-16a-methyl-17 oxopropyl-1,4-diene-3,20-dione to synthesize clobetasol propionate, 9a -Fluoro-11-hydroxy-16 a -methyl-17oxopropyl-1,4-diene-3,20-dione and lithium chloride mixture, mixed with dimethylformamide (DMF) in acetone The solution is refluxed for four days, the solution is moved to a vacuum, ethanol, methanol, and acetone are added, and the mixture is refluxed for another 4 days. Most of the solution is moved to a vacuum, water is added to the residue, the crude product is put into the ether solution, and the mixture is passed through with chloroform. The aluminum is purified by filtration and recrystallized with ethanol to produce clobetasol propionate as a raw material. Method I uses too much acetone, and there is a certain risk of operation.[0008] Process method 2 adopts 21-chloro-9a-fluoro-1I@ -hydroxy-16a-methyl-17_oxopropyl-4ene-3,20-dione to synthesize clobetasol propionate Cable. Dissolve 21-chloro-9 a -fluoro-11 P -hydroxy-16 a -methyl-17oxopropyl_4ene-3,20-dione in acetone, cool in an ice bath, and add slowly while stirring Chromic acid (prepared by chromic acid: add 53.3ml of concentrated sulfuric acid to 250ml of water and add 66.7g of chromium trioxide); 4 hours later, the mixture reaches room temperature, ether is added, and it is left for another 20 minutes. The mixture is washed with water, and then the solution is moved to a vacuum ; The residue is recrystallized with acetone-petroleum ether, which pollutes the environment. In the past, organic solvents were not safe for production operations. [0009] Chinese patent, application number 200610053511.5, provides a method of mixing betamethasone 17-propionate sulfonate and anhydrous lithium chloride in a ratio of 1:1 to 2 and dissolving in dimethylformamide ( DMF), the chlorination reaction is carried out; second, after the chlorination reaction is complete, it is separated by ice water, and then centrifuged to dry, after drying, the crude clobetasol propionate is obtained; third, the clobetasol propionate is crude The crude tasol is dissolved in methanol or ethanol, activated carbon is added, decolorized, filtered, and the activated carbon is recovered; fourth, the filtrate is concentrated under reduced pressure, crystallized, dehydrated, and dried to obtain the raw material of clobetasol propionate. It has the characteristics of easy availability of starting materials, simple reaction steps, less dangerous and harmful solvents, mature technology, and convenient industrial production.[0010] The process route is as follows:[0011]

[0012] Clobetasol propionate uses betamethasone as the starting material, goes through the steps of cyclic ester-hydrolysis-sulfonation-chlorination, and then undergoes rough refinement to obtain clobetasol propionate-a refined substance, and then undergoes dissolution , Filtration, concentration, cooling, centrifugation, and drying to obtain clobetasol propionate. But its process route is longer, there are many influencing factors, and there are many side reactions. Moreover, the solvents used are very polluting and difficult to recycle.

Example 1[0034] 20g of Betamethasone 17-ester obtained by cyclic ester hydrolysis reaction was dissolved in 150ml of acetone, and after fully stirring and dissolving, 6g of ZnCl2 was added, and the temperature was raised to 35°C, and then 30g of BTC was introduced, After the BTC is passed, the reaction is kept warm for 3 hours. After the reaction is completed, the temperature is 40°C, and the concentration is reduced under reduced pressure until the solution contains 30ml of acetone. Then 300ml of drinking water is added for water separation and filtration. After drying for 16 hours at °C, 19.64 g of crude clobetasol propionate was obtained. The yield was 98.2%, and the crude clobetasol propionate content was 96.9% after analysis.Example 2[0036] 20g of betamethasone 17-ester compound obtained by cyclic ester hydrolysis reaction was dissolved in 150ml of acetone, and after fully stirring and dissolving, 7.2g of FeCl3 was added, heated to 30°C, and then 24g of BTC was introduced After the BTC is passed, the reaction is kept warm for 2 hours. After the reaction is completed, the solution is concentrated under reduced pressure at a temperature of 35°C until the solution contains 20ml of acetone, and then 300ml of drinking water is added for water precipitation, filtered, and finally at the temperature After drying for 10 hours at 85°C, 19.5 g of crude clobetasol propionate was obtained. The yield was 97.5%, and the crude clobetasol propionate content was 95.6% after analysis.Example 3[0038] 20g of Betamethasone 17-ester obtained by the cyclic ester hydrolysis reaction was dissolved in 150ml of acetone, and after fully stirring and dissolving, 4g of AlCl3 was added and the temperature was raised to 35°C, and then 28g of BTC was introduced, After the BTC is passed through, the reaction is kept warm for 4 hours. After the reaction is completed, the temperature is 30°C, and concentrated under reduced pressure until the solution contains 20ml of acetone. Then 300ml of drinking water is added for water precipitation, filtered, and finally at a temperature of 75 After drying for 18 hours at °C, 19.62g crude clobetasol propionate was obtained. The yield was 98.1%, and the crude clobetasol propionate content was 95.8% after analysis.Example 4[0040] The betamethasone 17-ester 20g obtained by the cyclic ester hydrolysis reaction was dissolved in 100ml of acetone, and after being fully stirred to dissolve, 4g of ZnCl3 was added, heated to 40°C, and then passed into 25g of BTC, After the BTC is passed, the reaction is kept for 5 hours. After the reaction is completed, the temperature is 40°C, and the concentration is reduced under reduced pressure until the solution contains 20ml of acetone. Then 200ml of drinking water is added for water precipitation, filtered, and finally at a temperature of 80°C. After drying for 18 hours at °C, 19.54 g of crude clobetasol propionate was obtained. The yield was 97.7%, and the crude clobetasol propionate content was 96.2% after analysis.[0041] Example 5 [0042] 20g of Betamethasone 17-ester obtained by cyclic ester hydrolysis reaction was dissolved in 200ml of acetone, and after fully stirring and dissolving, 8g of ZnCl3 was added and the temperature was raised to 50°C. Then pass in 40g BTC. After passing the BTC, keep it warm and react for 3 hours. After the reaction is completed, perform vacuum concentration at a temperature of 40°C until the solution contains 40ml of acetone, and then add 400ml of drinking water for hydrolysis. Filter, and finally dry at 85°C for 18 hours to obtain 19.58 g of crude clobetasol propionate. The yield was 97.9%, and the crude clobetasol propionate content was 96.9% after analysis.Example 6[0044] 20g of betamethasone 17-ester compound obtained by cyclic ester hydrolysis reaction was dissolved in 80ml of acetone, and after fully stirring and dissolving, 6g of ZnCl3 was added, and after the temperature was raised to 40°C, 25g of BTC was introduced, After the BTC is passed, the reaction is kept for 3 hours. After the reaction is completed, it is concentrated under reduced pressure at a temperature of 40°C, and concentrated until the solution contains 10ml of acetone. Then 150ml of drinking water is added for water precipitation, filtered, and finally at a temperature of 85°C. After drying for 18 hours at °C, 19.3g crude clobetasol propionate was obtained. The yield was 96.5%, and the crude clobetasol propionate content was 96.5% after analysis.
Publication numberPriority datePublication dateAssigneeTitleUS3721687A *1968-01-191973-03-20Glaxo Lab Ltd3-keto-delta 4-9alpha-halo-11-oxygenated-16-methyl or methylene-17alpha-acyloxy-20-keto-21-halo pregnenesCN1923842A *2006-09-112007-03-07Zhejiang Dingtai Pharmaceutical Co., Ltd.Manufacturing method of clobetasol propionate
Publication numberPriority datePublication dateAssigneeTitleFamily To Family CitationsCN105646630A *2015-08-102016-06-08Shandong Taihua Biological Technology Co., Ltd.One-pot Preparation of Clobetasol Propionate IntermediateCN112110972A *2019-06-212020-12-22Henan Lihua Pharmaceutical Co., Ltd.A kind of preparation method of clobetasol propionateCN112028957A *2020-07-292020-12-04Henan Lihua Pharmaceutical Co., Ltd.A kind of clobetasol propionate intermediate and preparation method

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Medical uses
Clobetasol propionate is used for the treatment of various skin disorders including eczema, herpes labialis,[8] psoriasis, and lichen sclerosus. It is also used to treat several auto-immune diseases including alopecia areata, lichen planus (auto immune skin nodules), and mycosis fungoides (T-cell skin lymphoma). It is used as first-line treatment for both acute and chronic GVHD of the skin.[9]
Clobetasol propionate is used cosmetically by dark-skinned women for skin whitening, although this use is controversial. The U.S. Food and Drug Administration has not approved it for that purpose, and sales without a prescription are illegal in the U.S. Nonetheless, skin-whitening creams containing this ingredient can sometimes be found in ethnic beauty supply stores in New York City and on the internet. It is also sold internationally, and does not require a prescription in some countries. Whitening creams with clobetasol propionate, such as Hyprogel, can make skin thin and easily bruised, with visible capillaries, and acne. It can also lead to hypertension, elevated blood sugar, suppression of the body’s natural steroids, and stretch marks, which may be permanent.[10]
Clobetasol propionate is, along with mercury and hydroquinone, “amongst the most toxic and most used agents in lightening products.” Many products sold illegally have higher concentrations of clobetasol propionate than is permitted for prescription drugs.[11]
Contraindications
According to the California Environmental Protection Agency, clobetasol propionate should not be used by pregnant women, or women expecting to become pregnant soon, as studies with rats shows a risk of birth defects:[12]
“Studies in the rat following oral administration at dosage levels up to 50 mcg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose. Pregnancy: Teratogenic Effects (i.e., possibility of causing abnormalities in fetuses): Pregnancy Category C: Clobetasol propionate has not been tested for teratogenicity when applied topically; however, it is absorbed percutaneously, and when administered subcutaneously it was a significant teratogen in both the rabbit and mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent. There are no adequate and well-controlled studies of the teratogenic effects of clobetasol propionate in pregnant women. Temovate Cream and Ointment should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.”
Forms
Clobetasol propionate is marketed and sold worldwide under numerous names, including Clobex, Clob-x (Colombia), Clovate, Clobet (Biolab Thailand) Clonovate (T.O. Chemicals, Thailand), Cormax (Watson, US), Haloderm (Switzerland, by ELKO Org), Pentasol (Colombia), Cosvate, Clop (Cadila Healthcare, India), Propysalic (India), Temovate (US), Dermovate[13] (GlaxoSmithKline, Canada, Estonia, Pakistan, Switzerland, Portugal, Romania, Israel), Olux, ClobaDerm, Tenovate, Dermatovate, Butavate, Movate, Novate, Salac (Argentina), and Powercort, Lotasbat and Kloderma (Indonesia), Lemonvate (Italy), Delor (Ethiopia), Psovate (Turkey).
References
- ^ “Clobetasol Propionate Topical Ointment 0.05% Information – Drug Encyclopedia”. Kaiser Permanente.
- ^ Jump up to:a b c d e f “Clobetasol Propionate Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ Jump up to:a b c British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1210. ISBN 9780857113382.
- ^ “Clobetasol topical Use During Pregnancy”. Drugs.com. Retrieved 13 April 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 487. ISBN 9783527607495.
- ^ “The Top 300 of 2019”. ClinCalc. Retrieved 16 October 2021.
- ^ “Clobetasol – Drug Usage Statistics”. ClinCalc. Retrieved 16 October 2021.
- ^ Hull C, McKeough M, Sebastian K, Kriesel J, Spruance S (March 2009). “Valacyclovir and topical clobetasol gel for the episodic treatment of herpes labialis: a patient-initiated, double-blind, placebo-controlled pilot trial”. Journal of the European Academy of Dermatology and Venereology. 23 (3): 263–7. doi:10.1111/j.1468-3083.2008.03047.x. PMID 19143902. S2CID 205588376.
- ^ E. Fougera and Co. “CLOBETASOL PROPIONATE CREAM USP, 0.05% CLOBETASOL PROPIONATE OINTMENT USP, 0.05%<“. NIH Daily Med.
- ^ Saint Louis C (January 15, 2010). “Creams Offering Lighter Skin May Bring Risks”. New York Times.
- ^ Gbetoh MH, Amyot M (October 2016). “Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada”. Environmental Research. 150: 403–410. Bibcode:2016ER….150..403G. doi:10.1016/j.envres.2016.06.030. hdl:1866/19006. PMID 27372064.
- ^ Office of Environmental Health Hazard Assessment (August 22, 1997). “Chemicals Under Consideration For Possible Listing Via The “Formally Required To Be Labeled Or Identified” Mechanism”. California Environmental Protection Agency. Archived from the original on 2001-07-20. Retrieved 2007-05-06.
- ^ “DERMOVATE 0.05% W/V OINTMENT – Clobetasol Topical(0.05% w/v) Glaxo SmithKline Pharmaceuticals Ltd”. GNH. Retrieved 2021-07-16.
External links
- “Clobetasol propionate”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Pronunciation | /kloʊˈbeɪtəsɒl/[1] |
| Trade names | Temovate, Clobex, Cormax, others |
| AHFS/Drugs.com | Monograph |
| License data | US DailyMed: Clobetasol_propionate |
| Pregnancy category | AU: B3 |
| Routes of administration | Topical |
| ATC code | D07AD01 (WHO) |
| Legal status | |
| Legal status | In general: ℞ (Prescription only) |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 25122-46-7 |
| PubChem CID | 32798 |
| IUPHAR/BPS | 7062 |
| DrugBank | DB01013 |
| ChemSpider | 30399 |
| UNII | 779619577M |
| KEGG | D01272 |
| ChEBI | CHEBI:31414 |
| ChEMBL | ChEMBL1159650 |
| CompTox Dashboard (EPA) | DTXSID6045907 |
| ECHA InfoCard | 100.042.380 |
| Chemical and physical data | |
| Formula | C25H32ClFO5 |
| Molar mass | 466.97 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (verify) |
//////////////////Clobetasol propionate, CCI 4725, CCI-4725, GR 2/925, GR-2/925, Glucocorticoid, anti-inflammatory
[H][C@@]12C[C@H](C)[C@](OC(=O)CC)(C(=O)CCl)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C