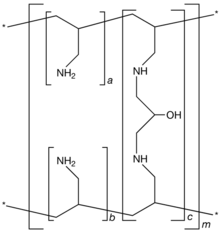

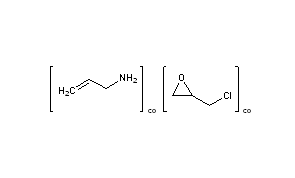
SEVELAMER
2-(chloromethyl)oxirane;prop-2-en-1-amine
CAS 52757-95-6, Molecular Formula, (C3-H7-N.C3-H5-Cl-O)x, Molecular Weight, 149.6198, HSDB 7608
Sevelamer hydrochloride [USAN]
152751-57-0, (C3-H7-N.C3-H5-Cl-O.Cl-H)x-, 186.0807, GT 16-026A
- A crosslinked polymeric amine that binds PHOSPHATES and BILE ACIDS; it is nonabsorbed; used for hyperphosphatemia during HEMODIALYSIS and in END-STAGE RENAL DISEASE; used like calcium acetate.
Sevelamer carbonate [USAN]
845273-93-0, (C3-H7-N.C3-H5-Cl-O)x-.x-C-H2-O3, 211.6436, GT335-012
- A polymeric amine that binds phosphate and is used to treat HYPERPHOSPHATEMIA in patients with kidney disease.
Drug Name:Sevelamer Carbonate,
Trade Name:Renvela®
MOA:Phosphate binder
Indication:Hyperphosphatemia
Company:Genzyme (Originator)
Sales:$1,037.9 Million (Y2015); 
$902.9 Million (Y2014);
$997.5 Million (Y2013);
$842.4 Million (Y2012);
$581 Million (Y2011);ATC Code:
Sevelamer Carbonate was first approved by the U.S. Food and Drug Administration (FDA) on Jul 19, 2007, then approved by European Medicine Agency (EMA) on Jun 21, 2009. However, till 2013, China Food and Drug Administration approved this drug. It was developed by Genzyme, and the trade name is Renvela®. On the other hand, US FDA firstly approved Sevelamer HCl (Renagel®) on Oct 30, 1998.
Renvela® is a non-absorbed phosphate binding crosslinked polymer, containing multiple amines separated by one carbon from the polymer backbone. These amines exist in a protonated form in the intestine and interact with phosphate molecules through ionic and hydrogen bonding. By binding phosphate in the gastrointestinal tract and decreasing absorption, sevelamer carbonate lowers the phosphate concentration in the serum (serum phosphorus).
Renvela® is available as film-coated tablet for oral use, containing 800 mg of free sevelamer carbonate on an anhydrous basis. The initial dose is 0.8 or 1.6 grams orally three times per day with meals.SevelamerCAS Registry Number: 52757-95-6
CAS Name: 2-Propen-1-amine polymer with (chloromethyl)oxirane
Additional Names: allylamine polymer with 1-chloro-2,3-epoxypropane; allylamine-epichlorohydrin copolymer; poly(allylamin-co-N,N¢-diallyl-1,3-diamino-2-hydroxypropane)
Literature References: Polymeric non-absorbed phosphate binder consisting of polyallylamine crosslinked with epichlorohydrin to form a hydrogel where 40% of the amines are protonated. Follows the general formula of (C3H7N.C3H5ClO)n. Binds dietary phosphate leading to increased fecal excretion, decreased absorption and decreased serum phosphorous levels. Prepd not claimed: S. R. Holmes-Farley et al.,WO9505184; eidem,US5496545 (1995, 1996 both to GelTex). Mechanism of action: S. R. Holmes-Farley et al.,J. Macromol. Sci. Pure Appl. Chem.A36, 1085 (1999). Determn of phosphate binding capacity: J. R. Mazzeo et al.,J. Pharm. Biomed. Anal.19, 911 (1999). Clinical studies in end stage renal disease: E. A. Slatopolsky et al.,Kidney Int.55, 299 (1999).
Derivative Type: Hydrochloride
CAS Registry Number: 152751-57-0
Manufacturers’ Codes: PB-94; GT16-026A
Trademarks: Renagel (Genzyme)
Properties: Insol in water. Hydrophilic.
Therap-Cat: Antihyperphosphatemic.
Keywords: Antihyperphosphatemic.
Sevelamer (rINN) is a phosphate binding medication used to treat hyperphosphatemia in patients with chronic kidney disease. When taken with meals, it binds to dietary phosphate and prevents its absorption. Sevelamer was invented and developed by GelTex Pharmaceuticals. Sevelamer is marketed by Sanofi under the brand names Renagel (sevelamer hydrochloride) and Renvela (sevelamer carbonate).
Chemistry and pharmacology
Sevelamer consists of polyallylamine that is crosslinked with epichlorohydrin.[1] The marketed form sevelamer hydrochloride is a partial hydrochloride salt being present as approximately 40% amine hydrochloride and 60% sevelamer base. The amine groups of sevelamer become partially protonated in the intestine and interact with phosphate ions through ionic and hydrogen bonding.
Medical uses
Sevelamer is used in the management of hyperphosphatemia in adult patients with stage 4 and 5 chronic kidney disease (CKD) on hemodialysis. Its efficacy at lowering phosphate levels is similar to that of calcium acetate, but without the accompanying risk of hypercalcemia and arterial calcification.[2][3] In patients with CKD, it has also been shown to reduce triglycerides and LDL, and increase HDL.[4]
This is a phosphate binder.
Contraindications
Sevelamer therapy is contraindicated in hypophosphatemia or bowel obstruction. In hypophosphatemia, sevelamer could exacerbate the condition by further lowering phosphate levels in the blood, which could be fatal.[5]
Adverse effects
Common adverse drug reactions (ADRs) associated with the use of sevelamer include: hypotension, hypertension, nausea and vomiting, dyspepsia, diarrhea, flatulence, and/or constipation.
Other effects
Sevelamer can significantly reduce serum uric acid.[6] This reduction has no known detrimental effect and several beneficial effects, including reducing hyperuricemia, uric acid nephrolithiasis, and gout.
Sevelamer is able to sequester advanced glycation end products (AGEs) in the gut, preventing their absorption into the blood. AGEs contribute to oxidative stress, which can damage cells (like beta cells, which produce insulin in the pancreas). As Vlassara and Uribarri explain in a 2014 review on AGEs, this may explain why sevelamer, but not calcium carbonate (a phosphate binder that does not sequester AGEs), has been shown to lower AGEs in the blood, as well as oxidative stress and inflammatory markers.[7]
SYN
https://www.oatext.com/polymer-and-heterocyclic-compounds-their-utility-and-application-as-drug.php
Sevelamer hydrochloride (Renagel VR ) was the first polymeric phosphate control and removal of excess phosphate is of benefit to patients with chronic kidney disease CKD where dialysis is unable to maintain safe phosphorus levels. Sevelamer limits the absorption of dietary phosphorus by binding phosphate in the intestine through ionic interaction with the polyamine polymer. Sevelamer is across linked form of poly(allylamine) containing primary and secondary aliphatic amine residues and was approved for the treatment of hyperphosphatemia by the FDA in 1998.The following Scheme 5 shows the synthetic lines for Sevelamer hydrochloride.

Scheme 5. Represent the formation of poly allylamine as a residue of sevelamer structure
Synthesis of Sevelamer Hydrochloride. Approximately 40 percent of the amine moieties are in the HCl form. Crosslinking degree is 10%.
SYN
- The synthesis of Sevelamer consists of crosslinkung poly(allylamine hydrochloride) with epichlorohydrin. The product is washed, dried and ground to the desired particle size to give the active substance.
Reference:1. US2009155368A1.Route 2
Reference:1. WO2011099038A2.
Reference:1. WO2010146603A1.Route 4
Reference:1. CN102796262A.
SYN
https://patents.google.com/patent/US20100331516A1/en
- The present invention relates to the process for preparation of carbonate salt of amine polymers, preferably Poly(allylamine-co-N,N′-diallyl-1,3-diamino-2-hydroxypropane)carbonate Formula-I, an antihyperphosphatemic agent.
- a, b=number of primary amine groups a+b=9
c=number of crosslinking groups c=1
m=large number to indicate extended polymer network
- Sevelamer carbonate is non-absorbable polymer marketed as Renvela
![™]() by Genzyme Corporation. It is known chemically as poly(allylamine-co-N,N′-diallyl-1,3-diamino-2-hydroxypropane) carbonate salt. It was developed as a pharmaceutical alternative to Sevelamer hydrochloride (Renagel®). Renvela
by Genzyme Corporation. It is known chemically as poly(allylamine-co-N,N′-diallyl-1,3-diamino-2-hydroxypropane) carbonate salt. It was developed as a pharmaceutical alternative to Sevelamer hydrochloride (Renagel®). Renvela![™]() contains Sevelamer carbonate, a non-absorbed phosphate binding crosslinked polymer, free of metal and calcium. It contains multiple amines separated by one carbon from the polymer backbone. These amines exist in a protonated form in the intestine and interact with phosphate molecules through ionic and hydrogen bonding. By binding phosphate in the dietary tract and decreasing absorption, Sevelamer carbonate lowers the phosphate concentration in the serum.
contains Sevelamer carbonate, a non-absorbed phosphate binding crosslinked polymer, free of metal and calcium. It contains multiple amines separated by one carbon from the polymer backbone. These amines exist in a protonated form in the intestine and interact with phosphate molecules through ionic and hydrogen bonding. By binding phosphate in the dietary tract and decreasing absorption, Sevelamer carbonate lowers the phosphate concentration in the serum. - [0004]
Sevelamer carbonate is an anion exchange resin with the same polymeric structure as Sevelamer hydrochloride in which carbonate replaces chloride as the counterion. While the counterions differ for the two salts, the polymer itself, the active moiety, is the same. The protonated amines can be indirectly measured as carbonate content in meq/gm. Renvela![™]() is used in End Stage Renal Disease (ESRD) which leads to hyperphosphatemia due to retention of phosphorous. This condition can lead to ectopic calcification. Renvela
is used in End Stage Renal Disease (ESRD) which leads to hyperphosphatemia due to retention of phosphorous. This condition can lead to ectopic calcification. Renvela![™]() binds dietary phosphate in GI tract and thus controls the serum phosphate levels. The potency of Renvela
binds dietary phosphate in GI tract and thus controls the serum phosphate levels. The potency of Renvela![™]() is measured in terms of its Phosphate Binding Capacity (PBC) by Phosphate Assay (PA). Treatment of hyperphosphatemia includes reduction in dietary intake of phosphate, inhibition of intestinal phosphate absorption with phosphate binders, and removal of phosphate with dialysis. Sevelamer carbonate taken with meals has been shown to control serum phosphorus concentrations in patients with CKD who are on dialysis. Currently Sevelamer hydrochloride is used to cure hyperphosphatemia. As a consequence ESRD patients still need a high dosage of Renagel® to meet clinical end-points, leading to adverse effect such as gastrointestinal discomfort and problems with patient compliance. But systemic acidosis development or worsening of pre-existing acidosis has been reported in many patients on long term dialysis who are given Sevelamer hydrochloride (Perit Dial Int. 2002, 22, 737-738, Nephron 2002, 92, 499-500, Kidney Int. 2004, 66, S39-S45, Ren. Fail 2005, 27,143-147).
is measured in terms of its Phosphate Binding Capacity (PBC) by Phosphate Assay (PA). Treatment of hyperphosphatemia includes reduction in dietary intake of phosphate, inhibition of intestinal phosphate absorption with phosphate binders, and removal of phosphate with dialysis. Sevelamer carbonate taken with meals has been shown to control serum phosphorus concentrations in patients with CKD who are on dialysis. Currently Sevelamer hydrochloride is used to cure hyperphosphatemia. As a consequence ESRD patients still need a high dosage of Renagel® to meet clinical end-points, leading to adverse effect such as gastrointestinal discomfort and problems with patient compliance. But systemic acidosis development or worsening of pre-existing acidosis has been reported in many patients on long term dialysis who are given Sevelamer hydrochloride (Perit Dial Int. 2002, 22, 737-738, Nephron 2002, 92, 499-500, Kidney Int. 2004, 66, S39-S45, Ren. Fail 2005, 27,143-147). - [0005]
Administration of Sevelamer hydrochloride adds to metabolic acid load because the resin removes some bicarbonate or bicarbonate precursor (mainly short chain fatty acid anions) from the body and replaces it with chloride. Each molecule of chloride contributed to the body in exchange for carbonate or bicarbonate precussor is equivalent to a molecule of hydrochloric acid added to the body, so the tendency of patients on long term haemodialysis to acidosis is inevitably increased when they take Sevelamer hydrochloride. (Kidney Int., 2005; 67: 776-777) - [0006]
This problem can be countered by an increase in the dialysate concentration of bicarbonate used in each dialysis session. A more fundamental solution, suitable for both dialyzed and non-dialyzed patients, would be the administration of Sevelamer free base, or any other suitable resin, not as the chloride but as body suitable counterion such as bicarbonate. Anion exchange resins have traditionally been synthesized in the chloride form, but the chloride in the current Sevelamer preparation is of no benefit to patients with renal failure. A change in the formulation of Sevelamer from its current chloride form to Sevelamer attached to bicarbonate would convert an acid load into a mild alkali load. (Cli. Sci. 1963; 24:187-200) - [0007]
U.S. Pat. No. 6,858,203 relates to phosphate-binding polymers provided for removing phosphate from the gastrointestinal tract. These polymers are useful for the treatment of hyperphosphatemia. - [0008]
WO 2006/050315 describes pharmaceutical compositions comprising a carbonate salt of an aliphatic amine polymer wherein the monovalent anion can prevent or ameliorate acidosis, in particular acidosis in patients with renal disease. - [0009]
HPLC Ion Chromatography PA method is used for the determination of PBC of Sevelamer HCl which can be adopted for determining the carbonate content from Sevelamer carbonate (J R Mazzeo et al, J. Pharm. Biomed. Anal. 19 (1999) 911-915). - [0010]
Our co-pending application number 1402/MUM/2006 dated 1 Sep. 2006 discloses process for preparation of Sevelamer HCl having phosphate binding capacity in the range of about 5.0 meq/gm to about 6.0 meq/gm and chloride content in the range of about 3.74 to about 5.60 meq/gm. - [0011]
The prior art mentioned above discussed advantages of Sevelamer carbonate over Sevelamer hydrochloride thus there remains need for commercially viable and industrially useful process for the preparation of Sevelamer carbonate having consistency in phosphate binding capacity, degree of cross linking, chloride content and carbonate content.
- The reaction is represented by the following reaction scheme:
- [0078]
100 gm Sevelamer hydrochloride was dispersed in 500 ml purified water and sodium hydroxide solution [20 gm sodium hydroxide dissolved in 500 ml purified water] was added to the obtained suspension followed by stirring at 25-35° C. for 30 minutes. The obtained material was filtered and wet cake was stirred in 1.0 L purified water for an hour. The material was filtered and cake was washed twice. Wet cake was dried at 50-90° C. for 5-6 hrs to get Sevelamer base (70 gm). LOD: 0.4% Chloride content: Nil.
Example 2
- [0079]
10 gm Sevelamer was suspended in 200 ml water and stirred. Carbon dioxide gas was purged into the obtained suspension at 25-35° C. for 8 hrs using dry ice. The obtained material was filtered and washed with 100 ml water [3×100] and the wet cake was dried on rotavapor at 90-95° C. to get Sevelamer carbonate (11.5 gm). Yield—115% w/w [Chloride content: 0.3%, Phosphate binding: 5.75 mMole/g, Carbonate content: 4.78 meq/g and Degree of crosslinking—16.4%], Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate.
Example 3
- [0080]
10 gm Sevelamer was added to 200 ml water and reacted with carbon dioxide gas under pressure at 25-35° C. for 7-8 hrs with stirring. The obtained material was filtered and washed with 100 ml water thrice [3×100]. The wet cake thus obtained was dried on rotavapor at 90-95° C. to get Sevelamer carbonate (11.3 gm). Yield—113% w/w Degree of crosslinking—16.4%, Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate.
Example 4
- [0081]
Sevelamer (7 gm) was added to 150 ml water and reacted with carbon dioxide gas under pressure at 60-65° C. for 7-8 hrs with stirring. The material obtained was filtered and washed with 100 ml purified water thrice [3×100]. The wet cake thus obtained was dried on rotavapor at 90-95° C. to get Sevelamer carbonate (9.3 gm). - [0082]
Yield—120% w/w Degree of crosslinking—16.4%, Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate.
Example 5
- [0083]
Sevelamer (7 gm) was added to 150 ml water and reacted with carbon dioxide gas by purging under pressure at 60-65° C. for 7-8 hrs with stirring. The material obtained was filtered and washed with 100 ml purified water thrice [3×100]. The wet cake thus obtained was dried on rotavapor at 90-95° C. to get Sevelamer carbonate (9.0 gm). - [0084]
[Degree of crosslinking—16.4%, Chloride content: 0.5%, Phosphate binding: 5.56 mMole/g and Carbonate content: 4.46 meq/g] Yield—110% w/w Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate.
Example 6
- [0085]
Sevelamer hydrochloride (10 gm) was treated Sodium hydroxide solution (2M) for 1 hr at temperature 25 to 35° C. to get Sevelamer base. Filter the free base and was added to 150 ml water and reacted with carbon dioxide gas by purging under pressure at 60-65° C. for 7-8 hrs with stirring. The material obtained was filtered and washed with 100 ml purified water thrice [3×100]. The wet cake thus obtained was dried on rotavapor under vacuum at 90-95° C. to get Sevelamer carbonate (9.3 gm). Yield—120% w/w. - [0086]
[Degree of crosslinking—16.4%, Chloride content: 0.2%, Phosphate binding: 5.45 mMole/g and Carbonate content: 4.36 meq/g]. - [0087]
Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate.
Example 7
- [0088]
Sevelamer hydrochloride (10 gm) was treated sodium hydroxide solution (2M) for 1 hr at temperature 25 to 35° C. to get Sevelamer base. Filter the free base and was added to 100 ml water. Sodium bicarbonate (10 gm dissolved in 1000 ml purified water) solution was added at temperature 60-65° C. for 4 hrs with stirring. Sevelamer Carbonate thus obtained was filtered and again subjected to for treatment of sodium bicarbonate solution (10 gm in 1000 ml). Reaction mixture was heated for 4 hrs at 60-65° C. with stirring. The material obtained was filtered and washed with 100 ml purified water thrice [3×100]. The wet cake thus obtained was dried under vacuum tray dryer at 80-90° C. for 24 hrs and further dried in atmospheric tray dryer at 100° C. for 36 hrs to get Sevelamer carbonate (9.0 gm). The loss of drying of material was about 5-7% achieved as per requirement. Yield—120% w/w, [Degree of crosslinking—16.4%, Chloride content: 0.01%, Phosphate binding: 5.68 mMole/g and Carbonate content: 4.85 meq/g]
Example 8
- [0089]
Sodium hydroxide pellets (41 gm) is dissolved in 600 ml methanol at 25-35° C. and polyallylamine hydrochloride (100 gm) is added to it followed by stirring for 5-6 hrs at temperature 25-35° C. The obtained reaction mass is filtered through hyflobed and filtrate is concentrated to reduce to half volume and the separated inorganic salt is filtered off over hyflobed. The obtained filtrate is concentrated completely under vacuum to get sticky mass (61 gm) of polyallylamine. Yield—61% w/w
Example 9
- [0090]
Polyallylamine (27.5 gm) dissolved in 100 ml water is charged into 1 L SS 316 autoclave and interacted with carbon dioxide gas under pressure (5.0 Kg/cm2). Initially 2-3 Kg/cm2 gas is consumed by the reaction mass and exotherm is observed from 28 C to 35° C. Then 5 Kg/cm2 pressure is maintained for 5-6 hours. After completion of the reaction the reaction mass is slowly added to 700 methanol and stirred for 3-4 hours. The separated solid (31 gm) is filtered, washed with 50 ml methanol and dried at 40-50° C. in vacuum oven. Yield—112% w/w
Example 10
- [0091]
Polyallylamine carbonate (20 gm) is dissolved in 30 ml water and cooled at 5-15° C. under stirring. The aqueous sodium hydroxide solution [dissolving 4.23 gm sodium hydroxide pellets into 4.2 ml of water] is added to reaction mass dropwise at 10-15° C. with continued stirring for 30 minutes. 101 ml toluene and 0.6 ml SPAN-85 is added to it and heated at 55-60° C. Epichlorohydrin (1.06 gm) is added to the reaction mass followed by stirring and heating for 3 hrs. The reaction mass is cooled at 25-35° C. and filtered through Buchner funnel. The obtained wet cake is added to 1 L acetone followed by stirring for 1 hour to get solid which was filtered through Buchner funnel. The aqueous organic washings are repeated for 7-10 times till polymer is free from excess alkalinity and the obtained wet cake is dried at 40-50° C. on rotavapor and then at 90-95° C. till constant weight of polymer is obtained (9 gm). Yield—45% w/w, Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate.
Example 11
- [0092]
Polyallylamine carbonate (20 gm) is dissolved in 30 ml water and cooled at 5-15° C. under stirring. The aqueous sodium hydroxide solution [dissolving 4.23 gm sodium hydroxide pellets into 4.2 ml of purified water] is added to obtained reaction mass dropwise at 10-15° C. with continued stirring for 30 minutes. 150 ml water and 0.6 ml SPAN-85 is added to it and heated at 60-80° C. Epichlorohydrin (1.06 gm) is added followed by stirring and heating is continued for 3 hours. The reaction mass is cooled at 25-35° C. and filtered through Buchner funnel. The obtained wet cake is added to 1 L acetone followed by stirring for 1 hour to get solid which is filtered through Buchner funnel. This aqueous organic washings are repeated for 7-10 times till the polymer is free from excess alkalinity and the obtained material is dried at 40-50° C. on rotavapor and/or Fluidised bed dryer then at 90-95° C. till constant weight of polymer is obtained (9 gm).
Example 12
- [0093]
Polyallylamine carbonate (20 gm) is dissolved in 30 ml water and cooled at 5-15° C. under stirring. The aqueous sodium hydroxide solution [dissolving 4.23 gm sodium hydroxide pellets into 4.2 ml of purified water] is added to the obtained reaction mass dropwise at 10-15° C. with continued stirring for 30 minutes. 150 ml water and 0.6 ml SPAN-85 is added to it and heated at 60-80° C. Epichlorohydrin (1.06 gm) is added followed by stirring and heating is continued for 3 hours. The reaction mass is cooled at 25-35° C. and filtered through Buchner funnel. The obtained wet cake is added to 1 L isopropyl alcohol (IPA) followed by stirring for 1 hour to get solid which is filtered through Buchner funnel. The obtained material is washed with water and organic solvents for 4-5 times till the polymer is free from excess alkalinity. The obtained wet cake is dried under vacuum tray dryer at 80-90° C. for 24 hrs and further dried in atmospheric tray dryer at 100° C. for 36 hrs till constant weight of dried polymer is obtained (15 gm). The loss on drying of material is around 6% as per requirement.
Example 13
- [0094]
In 1 L SS 316 autoclave, 75 gm allylamine and 200 ml water is charged and carbon dioxide gas under pressure (5 Kg/cm2) is purged into autoclave for 3-4 hours followed by stirring. Nitrogen gas is purged for 15 minutes. 9.8 gm VA-086 is added to the reaction mass and stirred at 70-80° C. for 12 hours and this solution is added to 1 L methanol under stirring. The separated material is filtered and washed with 100 ml methanol, suck dried and dried in vacuum oven at 50-60° C. to get 90 gm of polyallylamine carbonate. Yield—120% w/w
Example 14
- [0095]
Polyallylamine carbonate (20 gm) dissolved in 30 ml water is cooled at 5-15° C. under stirring and sodium hydroxide solution [dissolving 4.23 gm sodium hydroxide pellets into 4.2 ml of purified water] is added to the obtained reaction mass dropwise at 10-15° C. followed by continued stirring for 30 minutes. 101 ml toluene and 0.6 ml SPAN-85 is added to it and heated at 55-60° C. Epichlorohydrin (1.06 gm) is added and reaction mass is stirred and heated for 3 hours. Then it is cooled to 25-35° C. and filtered through Buchner funnel. The wet cake obtained is added to 1 to 1.5 L acetone followed by stirring for 1 hour to get solid which is filtered through Buchner funnel. The washings are repeated for 7-10 times till polymer is free from excess alkalinity. Wet cake (9 gm) is dried at 40-50° C. on rotavapor and then at 90-95° C. till constant weight of polymer is obtained. Yield—45% w/w
Example 15
- [0096]
Sevelamer hydrochloride (10 gm) was added to 10% aqueous sodium bicarbonate solution at 25-35° C. and stirred for 7-8 hrs. The material obtained was filtered and washed with 100 ml purified water thrice and the wet cake was dried on rotavapor at 90-95° C. to get Sevelamer carbonate (7.5 gm). Yield—75% w/w - [0097]
Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate. - [0098]
[Chloride content: 0.4%, Phosphate binding: 5.45 mMole/g and Carbonate content: 4.85 meq/g]
Example 16
- [0099]
Sevelamer hydrochloride (10 gm) was added to 10% aqueous sodium bicarbonate solution. The mixture was stirred at 60-65° C. for 4 hrs. The material obtained was filtered and the obtained wet cake was again subjected to the treatment of 10% sodium bicarbonate solution. Reaction mixture was heated for 4 hrs at 60-65° C. with stirring. The material obtained was filtered and washed with 100 ml purified water four times and the wet cake was dried on rotavapor under vacuum at 90-95° C. to get Sevelamer carbonate (7.5 gm). Yield—75% w/w, Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate, [Chloride content: 0.03%, Phosphate binding: 5.25 mMole/g and Carbonate content: 4.65 meq/g].
Example 17
- [0100]
Sevelamer hydrochloride (10 gm) was added into 130 ml solution of sodium bicarbonate (10 gm NaHCO3 in 130 ml water) and the mixture was stirred at 60-65° C. for 4 hrs. The material was filtered using Buckner funnel assembly. The obtained wet cake was added into 130 ml solution of sodium bicarbonate (10 gm NaHCO3 in 130 ml water) and stirred at 60-65° C. for 4 hrs. The material was filtered using Buckner funnel assembly and the wet cake was washed by stirring it in 100 ml water for 1 hr at 60-65° C. The material was filtered using Buckner funnel assembly. The wet cake was washed twice at 60-65° C. and dried on rotavapor at 90-95° C. to get Sevelamer carbonate (8.5 gm). Yield—75% w/w, Chloride content: 0.03%
Example 18
- [0101]
Sevelamer hydrochloride (1.1 Kg) was added into 15.5 L solution of sodium bicarbonate (1.1 Kg NaHCO3 in 14.3 L water). The obtained mixture was stirred at 60-65° C. for 4 hrs. The obtained material was filtered by centrifuge filter. The obtained wet cake was added into 15.5 L solution of sodium bicarbonate (1.1 Kg NaHCO3 in 14.3 L water) and maintained stirring at 60-65° C. for 4 hrs. The material was filtered by centrifuge filter assembly and obtained wet cake was stirred in 11 L water for 1 hr at 60-65° C. The material was filtered by centrifuge filter and the washing of wet cake was repeated at 60-65° C. for two more times. The obtained wet cake was dried in air tray dryer (ATD) at 90-100° C. for 30-36 hrs and LOD was checked after every five hours till LOD was in the range of 5 to 10%. to get Sevelamer carbonate (0.995 Kg), [Chloride content: 0.03%, Phosphate binding capacity: 5.5 mmole/gm, Carbonate content: 5.1 meq/gm]
Example 19
- [0102]
Sevelamer hydrochloride (10 gm) was added to sodium bicarbonate solution (10 gm in 200 ml) at 25-35° C. The reaction mixture was heated for 4 hrs at 60-65° C. with stirring. Sevelamer Carbonate thus obtained was filtered and again subjected to treatment of Sodium bicarbonate solution (10 gm in 200 ml). Reaction mixture was heated for 4 hrs at 60-65° C. with stirring. The material was filtered off and washed with 100 ml purified water four times (4×100 ml) and the wet cake was dried under vacuum tray dryer at 80-90° C. for 24 hrs and further dried in atmospheric tray dryer at 100° C. for 36 hrs till constant weight of dried polymer was obtained. The loss on drying of material was around 6% (Limit: 4-10%), achieved as per requirement. Sevelamer carbonate (7.5 gm) was obtained which can be sieved through 30 mesh for uniformity of the sample. Yield—75% w/w. Solid state 13C NMR shows prominent peak at 164 ppm which is for carbon of carbonate. [Chloride content: 0.02%, Phosphate binding: 5.56 mMole/g and Carbonate content: 4.74 meq/g].
Example 20
- [0103]
10 g wet cake of Sevelamer carbonate was subjected to drying in air tray dryer at 80-100° C. at atmospheric pressure for 36 hours and LOD was measured after every five hours. LOD: 7.5% Yield: 3.1 gm
Example 21
- [0104]
100 g wet cake of Sevelamer carbonate was subjected to drying in air tray dryer at 80-100° C. at atmospheric pressure for 37 hours and LOD was measured. LOD: 8.4% Yield: 30 gm
Example 22
- [0105]
10 g wet cake of Sevelamer carbonate was subjected to drying in vacuum tray dryer at 50-100° C. at reduced pressure for 24 hours and LOD was measured. LOD: 8.5% Yield: 3.2 gm
Example 23
- [0106]
100 g wet cake of Sevelamer carbonate was subjected to drying in vacuum tray dryer at 50-100° C. at reduced pressure for 24 hours and LOD was measured. LOD: 8.9% Yield: 31 gm
Example 24
- [0107]
10 Kg wet cake of Sevelamer carbonate was subjected to drying in fluidized bed dryer at 80-100° C. for 16 hours and LOD was measured after every five hours. LOD: 7.9% Yield: 3.4 kg
Example 25
- [0108]
15 Kg wet cake of Sevelamer carbonate was subjected to drying in fluidised bed dryer at 80-110° C. for 16 hours and LOD was measured. LOD: 8.8% Yield: 4.9 kg.
Example 26
- [0109]
10 g wet cake of Sevelamer carbonate was subjected to drying in rotary evaporator at 50-100° C. at reduced pressure for 16 hours and LOD was measured after every five hours. - [0110]
LOD: 9.1% Yield: 3.1 gm
Example 27
- [0111]
100 g wet cake of polyallylamine carbonate is subjected to drying in rotary evaporator at 50-100° C. at reduced pressure for 16 hours and LOD is measured. LOD: 8.9% Yield: 33 gm.
SYN
https://patents.google.com/patent/CN102675510A/enHyperphosphatemia is a kind of patient’s disease on one’s body that often appears at renal tubal dysfunction, hypothyroidism, acute acromegaly or phosphoric acid salt drug overdose; Its treatment is normal adopts the pharmacotherapy of regimen or oral phosphorus adsorbent to carry out; But it has been generally acknowledged that the regimen effect is relatively poor, the use of phosphorus adsorbent is essential.In recent years; Discover that the compound that contains the polyallylamine structure has good phosphorus adsorptive power (as: USP 5496545,20040191212, Chinese patent 95193521.6 etc.), crosslinked polyallylamine class medicine is evident in efficacy especially; Wherein, SEVELAMER (Sevelamer) is because of its good clinical manifestation, and granted listing is used to treat hyperphosphatemia.The structural formula of SEVELAMER is following:
Synthesizing of crosslinked polyallylamine class medicine SEVELAMER (Sevelamer); Be to react by polyallylamine hydrochloride and linking agent; Different because of used linking agent, the phosphorus adsorptive power of product has than big-difference, and the most option table chloropharin of the present document of reporting is a linking agent.For example: in USP 5496545; Introduced the synthetic of a series of crosslinked allyl amine polymers oral, that the phosphorus adsorptive power is arranged, listed linking agent has: Epicholorohydrin, 1,4-butanediol diglycidyl ether, 1; 2-ethylene glycol bisthioglycolate glycidyl ether, 1; 3-propylene dichloride, 1,2-ethylene dichloride, 1,3-dibromopropane, 1; 2-ethylene dibromide, succinyl dichloride, dimethyl succinate salt, TDI, acrylate chloride and pyromellitic dianhydride, preferred cross-linking agents is an Epicholorohydrin.U.S. Pat 5496545 described SEVELAMER building-up processes are: in the alkaline aqueous solution, polyallylamine hydrochloride and Epicholorohydrin carry out crosslinking reaction under room temperature, react agglutination thing after 18 hours; Pour into and make its curing in the Virahol; Filter, repeatedly washing back redispersion is in water, after the filtration; Be scattered in a large amount of Virahols, obtain product through filtration, drying.The required reaction times of this technology is longer, and multiple times of filtration and washing in the operating process need be used a large amount of organic solvents, and in the series product, the highest phosphorus adsorptive power is 3.1mmol/g.Embodiment 1: sevelamer hydrochloride syntheticIn the 500mL flask; Add 46.2g (0.374mol) PAH hydrochloride, the 108.0mL deionized water dissolving adds sodium hydroxide and regulates pH=10-11; Drip the toluene solution of 11.0g (0.042mol) 1-chloro-3-p-toluenesulfonyl-2-propyl alcohol, heat up in 70-75 ℃ of reaction 4 hours.Reaction finishes, and adds hydrochloric acid and regulates pH=1-2, filters, and obtains the sevelamer hydrochloride bullion.The sevelamer hydrochloride bullion is scattered in the 300.0mL deionized water, and hydro-oxidation sodium is regulated pH=10.0-11.0, filters; Deionized water wash; The gained white solid through pulverizing, gets product sevelamer hydrochloride 38.8g again 70 ℃ of vacuum-dryings 8 hours; The phosphorus adsorptive value is 5.5mmol/g, and chloride ion content is 16.5%.Embodiment 2: carbonic acid SEVELAMER syntheticIn the 500mL flask; Add 150.0g (0.404mol; Weight concentration is 30%) the PAH hydrochloride aqueous solution; Add sodium hydroxide and regulate pH=10-11, drip the acetonitrile solution of 13.0g (0.042mol) 1-bromo-3-p-toluenesulfonyl-2-propyl alcohol, heat up in 70-75 ℃ of reaction 4 hours.Reaction finishes, and adds hydrochloric acid and regulates pH=1-2, filters, and obtains the sevelamer hydrochloride bullion.The sevelamer hydrochloride bullion is scattered in the 300.0mL deionized water, adds yellow soda ash and regulate pH=8.5-9.5, filter; Deionized water wash, gained white solid are 70 ℃ of vacuum-dryings 8 hours, again through pulverizing; Get product carbonic acid SEVELAMER 36.4g, the phosphorus adsorptive value is 5.4mmol/g.Embodiment 3: carbonic acid SEVELAMER syntheticIn the 500mL flask; Add 46.2g (0.374mol) PAH hydrochloride, the 108.0mL deionized water dissolving adds sodium hydroxide and regulates pH=10-11; Drip the toluene solution of 7.9g (0.042mol) 1-chloro-3-methylsulfonyl-2-propyl alcohol, heat up in 70-75 ℃ of reaction 4 hours.Reaction finishes, and adds hydrochloric acid and regulates pH=1-2, filters, and obtains the sevelamer hydrochloride bullion.The sevelamer hydrochloride bullion is scattered in the 300.0mL deionized water, and hydro-oxidation sodium is regulated pH=12.0-12.5, feeds dioxide gas to saturated; Filter; Deionized water wash, gained white solid are 70 ℃ of vacuum-dryings 8 hours, again through pulverizing; Get product carbonic acid SEVELAMER 40.5g, the phosphorus adsorptive value is 5.0mmol/g.

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
References
- ^ Ramsdell R (June 1999). “Renagel: a new and different phosphate binder”. review. ANNA Journal. 26 (3): 346–7. PMID 10633608.
- ^ Burke SK (September 2000). “Renagel: reducing serum phosphorus in haemodialysis patients”. review. Hospital Medicine. 61 (9): 622–7. doi:10.12968/hosp.2000.61.9.1419. PMID 11048603.
- ^ Habbous S, Przech S, Acedillo R, Sarma S, Garg AX, Martin J (January 2017). “The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: a systematic review and meta-analysis”. review. Nephrology, Dialysis, Transplantation. 32 (1): 111–125. doi:10.1093/ndt/gfw312. PMID 27651467.
- ^ Patel L, Bernard LM, Elder GJ (February 2016). “Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials”. review. Clinical Journal of the American Society of Nephrology. 11 (2): 232–44. doi:10.2215/CJN.06800615. PMC 4741042. PMID 26668024.
- ^ Emmett M (September 2004). “A comparison of clinically useful phosphorus binders for patients with chronic kidney failure”. review. Kidney International Supplements. 66 (90): S25–32. doi:10.1111/j.1523-1755.2004.09005.x. PMID 15296504.
- ^ Locatelli F, Del Vecchio L (May 2015). “Cardiovascular mortality in chronic kidney disease patients: potential mechanisms and possibilities of inhibition by resin-based phosphate binders”. review. Expert Review of Cardiovascular Therapy. 13 (5): 489–99. doi:10.1586/14779072.2015.1029456. PMID 25804298. S2CID 32586527.
- ^ Vlassara H, Uribarri J (January 2014). “Advanced glycation end products (AGE) and diabetes: cause, effect, or both?”. review. Current Diabetes Reports. 14 (1): 453. doi:10.1007/s11892-013-0453-1. PMC 3903318. PMID 24292971.
External links
- “Sevelamer”. Drug Information Portal. U.S. National Library of Medicine.
- “Sevelamer hydrochloride”. Drug Information Portal. U.S. National Library of Medicine.
- “Sevelamer carbonate”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Pronunciation | (/sɛˈvɛləmər/ or /sɛˈvɛləmɪər/) |
| Trade names | Renagel, Renvela |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601248 |
| License data | EU EMA: by INNUS DailyMed: Sevelamer |
| Pregnancy category | AU: B3 |
| Routes of administration | By mouth |
| ATC code | V03AE02 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)US: ℞-onlyEU: Rx only |
| Pharmacokinetic data | |
| Bioavailability | 0% |
| Excretion | Feces 100% |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 52757-95-6 |
| PubChem CID | 3085017 |
| DrugBank | DB00658 |
| ChemSpider | 2341997 |
| UNII | 941N5DUU5C |
| KEGG | D08512 as HCl: D01983 |
| ChEMBL | ChEMBL1201492 |
| CompTox Dashboard (EPA) | DTXSID80872282 |
| Chemical and physical data | |
| Formula | [(C3H7N)a+b.(C9H17N2O)c]m where a+b:c = 9:1 |
| Molar mass | variable |
| (what is this?) (verify) |
//////////////////////SEVELAMER, HSDB 7608, GT335-012, GT 16-026A, PB 94, Antihyperphosphatemic

 by Genzyme Corporation. It is known chemically as poly(allylamine-co-N,N′-diallyl-1,3-diamino-2-hydroxypropane) carbonate salt. It was developed as a pharmaceutical alternative to Sevelamer hydrochloride (Renagel®). Renvela
by Genzyme Corporation. It is known chemically as poly(allylamine-co-N,N′-diallyl-1,3-diamino-2-hydroxypropane) carbonate salt. It was developed as a pharmaceutical alternative to Sevelamer hydrochloride (Renagel®). Renvela