
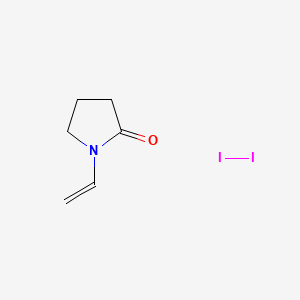
Povidone-iodine
PVP 1
UNII85H0HZU99M, BETADINE
CAS number 25655-41-8, Molecular Formula, (C6-H9-N-O)x-.x-I2, Molecular Weight, 364.9431
1-ethenylpyrrolidin-2-one;molecular iodine Povidone-Iodine
CAS Registry Number: 25655-41-8
CAS Name: 1-Ethenyl-2-pyrrolidinone homopolymer compd with iodine
Additional Names: 1-vinyl-2-pyrrolidinone polymers, iodine complex; iodine-polyvinylpyrrolidone complex; polyvinylpyrrolidone-iodine complex; PVP-I
Trademarks: Betadine (Purdue Frederick); Betaisodona (Mundipharma); Braunol (Braun Melsungen); Braunosan H (Braun Melsungen); Disadine D.P. (Stuart); Efodine (Fougera); Inadine (J & J); Isodine (Blair); Proviodine (Rougier); Traumasept (Wolff)
Literature References: An iodophor, q.v., prepd by Beller, Hosmer, US2706701; Hosmer, US2826532; Siggia, US2900305 (1955, 1958, and 1959, all to GAF). Prepn, history and use: Shelanski, Shelanski, J. Int. Coll. Surg.25, 727 (1956).
Properties: Yellowish-brown, amorphous powder with slight characteristic odor. Aq solns have a pH near 2 and may be made more neutral (but less stable) by the addition of sodium bicarbonate. Sol in alc, water. Practically insol in chloroform, carbon tetrachloride, ether, solvent hexane, acetone. Solns do not give the familiar starch test when freshly prepared.
Therap-Cat: Anti-infective (topical).
Therap-Cat-Vet: Anti-infective (topical).
Keywords: Antiseptic/Disinfectant; Halogens/Halogen Containing Compounds.
- An iodinated polyvinyl polymer used as topical antiseptic in surgery and for skin and mucous membrane infections, also as aerosol. The iodine may be radiolabeled for research purposes.
Povidone-iodine is a stable chemical complex of polyvinylpyrrolidone (povidone, PVP) and elemental iodine. It contains from 9.0% to 12.0% available iodine, calculated on a dry basis. This unique complex was discovered in 1955 at the Industrial Toxicology Laboratories in Philadelphia by H. A. Shelanski and M. V. Shelanski. During in vitro testing to demonstrate anti-bacterial activity it was found that the complex was less toxic in mice than tincture of iodine. Human clinical trials showed the product to be superior to other iodine formulations. Povidone-iodine was immediately marketed, and has since become the universally preferred iodine antiseptic.
Povidone-iodine (PVP-I), also known as iodopovidone, is an antiseptic used for skin disinfection before and after surgery.[1][2] It may be used both to disinfect the hands of healthcare providers and the skin of the person they are caring for.[2] It may also be used for minor wounds.[2] It may be applied to the skin as a liquid or a powder.[2]
Side effects include skin irritation and sometimes swelling.[1] If used on large wounds, kidney problems, high blood sodium, and metabolic acidosis may occur.[1] It is not recommended in women who are less than 32 weeks pregnant or are taking lithium.[2] Frequent use is not recommended in people with thyroid problems.[2] Povidone-iodine is a chemical complex of povidone, hydrogen iodide, and elemental iodine.[3] It contains 10% Povidone, with total iodine species equaling 10,000 ppm or 1% total titratable iodine.[3] It works by releasing iodine which results in the death of a range of microorganisms.[1]
Povidone-iodine came into commercial use in 1955.[4] It is on the World Health Organization’s List of Essential Medicines.[5] Povidone-iodine is available over the counter.[6] It is sold under a number of brand names including Betadine.[2]

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
Medical uses
Wound area covered in povidone-iodine. Gauze has also been applied.
Povidone-iodine is a broad spectrum antiseptic for topical application in the treatment and prevention of wound infection. It may be used in first aid for minor cuts, burns, abrasions and blisters. Povidone-iodine exhibits longer lasting antiseptic effects than tincture of iodine, due to its slow absorption via soft tissue, making it the choice for longer surgeries. Chlorhexidine provides superior results with equivalent adverse events.[7]
Consequently, PVP-I has found broad application in medicine as a surgical scrub; for pre- and post-operative skin cleansing; for the treatment and prevention of infections in wounds, ulcers, cuts and burns; for the treatment of infections in decubitus ulcers and stasis ulcers; in gynecology for vaginitis associated with candidal, trichomonal or mixed infections. For these purposes PVP-I has been formulated at concentrations of 7.5–10.0% in solution, spray, surgical scrub, ointment, and swab dosage forms; however, use of 10% povidone-iodine though recommended, is infrequently used, as it is poorly accepted by health care workers and is excessively slow to dry.[8][9]
Because of these critical indications, only sterile povidone-iodine should be used in most cases. Non-sterile product can be appropriate in limited circumstances in which people have intact, healthy skin that will not be compromised or cut. The non-sterile form of Povidone iodine has a long history of intrinsic contamination with Burkholderia cepacia (aka Pseudomonas cepacia), and other opportunistic pathogens. Its ability to harbor such microbes further underscores the importance of using sterile products in any clinical setting. Since these bacteria are resistant to povidone iodine, statements that bacteria do not develop resistance to PVP-I,[10] should be regarded with great caution: some bacteria are intrinsically resistant to a range of biocides including povidone-iodine.[11]
Antiseptic activity of PVP-I is because of free iodine (I2) and PVP-I only acts as carrier of I2 to the target cells. Most commonly used 10% PVP-I delivers about 1-3 ppm of I2 in a compound of more than 31,600 ppm of total iodine atoms. All the toxic and staining effects of PVP-I is due to the inactive iodine only.
Eyes
A buffered PVP-I solution of 2.5% concentration can be used for prevention of neonatal conjunctivitis, especially if it is caused by Neisseria gonorrhoeae, or Chlamydia trachomatis. It is currently unclear whether PVP-I is more effective in reducing the number of cases of conjunctivitis in neonates over other methods.[12] PVP-I appears to be very suitable for this purpose because, unlike other substances, it is also efficient against fungi and viruses (including HIV and Herpes simplex).[13]
Pleurodesis
It is used in pleurodesis (fusion of the pleura because of incessant pleural effusions). For this purpose, povidone-iodine is equally effective and safe as talc, and may be preferred because of easy availability and low cost.[14]
Alternatives
There is strong evidence that chlorhexidine and denatured alcohol used to clean skin prior to surgery is better than any formulation of povidone-iodine[7]
Contraindications
PVP-I is contraindicated in people with hyperthyroidism (overactive thyroid gland) and other diseases of the thyroid, after treatment with radioiodine, and in people with dermatitis herpetiformis[why?] (Duhring’s disease).[15]
Side effects
The sensitization rate to the product is 0.7%.[16]
Interactions
The iodine in PVP-I reacts with hydrogen peroxide, silver, taurolidine and proteins such as enzymes, rendering them (and itself) ineffective. It also reacts with many mercury compounds, giving the corrosive compound mercury iodide, as well as with many metals, making it unsuitable for disinfecting metal piercings.[15]
Iodine is absorbed into the body to various degrees, depending on application area and condition of the skin. As such, it interacts with diagnostic tests of the thyroid gland such as radioiodine diagnostics, as well as with various diagnostic agents used on the urine and stool, for example Guaiacum resin.[15]
Structure
Structure of povidone-iodine complex.
Povidone-iodine is a chemical complex of the polymer povidone (polyvinylpyrrolidone) and triiodide (I3−).[17]
It is soluble in cold and mild-warm water, ethyl alcohol, isopropyl alcohol, polyethylene glycol, and glycerol. Its stability in solution is much greater than that of tincture of iodine or Lugol’s solution.
Free iodine, slowly liberated from the povidone-iodine (PVP-I) complex in solution, kills cells through iodination of lipids and oxidation of cytoplasmic and membrane compounds. This agent exhibits a broad range of microbiocidal activity against bacteria, fungi, protozoa, and viruses. Slow release of iodine from the PVP-I complex in solution minimizes iodine toxicity towards mammalian cells.
PVP-I can be loaded into hydrogels, which can be based on carboxymethyl cellulose (CMC), poly(vinyl alcohol) (PVA), and gelatin, or on crosslinked polyacrylamide. These hydrogels can be used for wound dressing. The rate of release of the iodine in the PVP-I is heavily dependent on the hydrogel composition: it increases with more CMC/PVA and decreases with more gelatin.
History
PVP-I was discovered in 1955, at the Industrial Toxicology Laboratories in Philadelphia by H. A. Shelanski and M. V. Shelanski.[18] They carried out tests in vitro to demonstrate anti-bacterial activity, and found that the complex was less toxic in mice than tincture of iodine. Human clinical trials showed the product to be superior to other iodine formulations.[19]
Following the discovery of iodine by Bernard Courtois in 1811, it has been broadly used for the prevention and treatment of skin infections, as well as the treatment of wounds. Iodine has been recognized as an effective broad-spectrum bactericide, and is also effective against yeasts, molds, fungi, viruses, and protozoans. Drawbacks to its use in the form of aqueous solutions include irritation at the site of application, toxicity, and the staining of surrounding tissues. These deficiencies were overcome by the discovery and use of PVP-I, in which the iodine is carried in a complexed form and the concentration of free iodine is very low. The product thus serves as an iodophor.
Research
Schematic of povidone-iodine complex wrapping a single wall carbon nanotube (black).[20]
Povidone-iodine has found application in the field of nanomaterials. A wound-healing application has been developed which employs a mat of single wall carbon nanotubes (SWNTs) coated in a monolayer of povidone-iodine.[20]
Research has previously found that the polymer polyvinylpyrrolidone (PVP, povidone) can coil around individual carbon nanotubes to make them water-soluble.[21]
References
- ^ Jump up to:a b c d World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 321–323. hdl:10665/44053. ISBN 9789241547659.
- ^ Jump up to:a b c d e f g British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 840. ISBN 9780857111562.
- ^ Jump up to:a b Encyclopedia of polymer science and technology (3 ed.). John Wiley & Sons. 2013. p. 728. ISBN 9780470073698. Archived from the original on 2017-01-13.
- ^ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 68. ISBN 9780470015520. Archived from the original on 2017-01-13.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06.
- ^ “Povidone/iodine solution: Indications, Side Effects, Warnings – Drugs.com”. http://www.drugs.com. Archived from the original on 13 January 2017. Retrieved 11 January 2017.
- ^ Jump up to:a b Wade RG, Burr NE, McCauley G, Bourke G, Efthimiou O (September 2020). “The Comparative Efficacy of Chlorhexidine Gluconate and Povidone-iodine Antiseptics for the Prevention of Infection in Clean Surgery: A Systematic Review and Network Meta-analysis”. Annals of Surgery. Publish Ahead of Print. doi:10.1097/SLA.0000000000004076. PMID 32773627.
- ^ Slater K, Cooke M, Fullerton F, Whitby M, Hay J, Lingard S, et al. (September 2020). “Peripheral intravenous catheter needleless connector decontamination study-Randomized controlled trial”. American Journal of Infection Control. 48 (9): 1013–1018. doi:10.1016/j.ajic.2019.11.030. PMID 31928890.
- ^ Slater K, Fullerton F, Cooke M, Snell S, Rickard CM (September 2018). “Needleless connector drying time-how long does it take?”. American Journal of Infection Control. 46 (9): 1080–1081. doi:10.1016/j.ajic.2018.05.007. PMID 29880433. S2CID 46968733.
- ^ Fleischer W, Reimer K (1997). “Povidone-iodine in antisepsis–state of the art”. Dermatology. 195 Suppl 2 (Suppl 2): 3–9. doi:10.1159/000246022. PMID 9403248.
- ^ Rose H, Baldwin A, Dowson CG, Mahenthiralingam E (March 2009). “Biocide susceptibility of the Burkholderia cepacia complex”. The Journal of Antimicrobial Chemotherapy. 63 (3): 502–10. doi:10.1093/jac/dkn540. PMC 2640157. PMID 19153076.
- ^ Martin I, Sawatzky P, Liu G, Mulvey MR (February 2015). “Neisseria gonorrhoeae in Canada: 2009-2013”. Canada Communicable Disease Report. 41 (2): 35–41. doi:10.1002/14651858.CD001862.pub3. PMC 6457593.
- ^ Najafi Bi R, Samani SM, Pishva N, Moheimani F (2003). “Formulation and Clinical Evaluation of Povidone-Iodine Ophthalmic Drop”. Iranian Journal of Pharmaceuticical Research. 2 (3): 157–160.
- ^ Agarwal R, Khan A, Aggarwal AN, Gupta D (March 2012). “Efficacy & safety of iodopovidone pleurodesis: a systematic review & meta-analysis”. The Indian Journal of Medical Research. 135: 297–304. PMC 3361864. PMID 22561614.
- ^ Jump up to:a b c Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 983–5. ISBN 978-3-85200-181-4.
- ^ Niedner R (1997). “Cytotoxicity and sensitization of povidone-iodine and other frequently used anti-infective agents”. Dermatology. 195 Suppl 2 (Suppl 2): 89–92. doi:10.1159/000246038. PMID 9403263.
- ^ Kutscher, Bernhard (2020). “Dermatologicals (D), 4. Antiseptics and Disinfectants (D08), Anti‐Acne Preparations (D10), and Other Dermatological Preparations (D11)”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–22. doi:10.1002/14356007.w08_w03.
- ^ U.S. Patent 2,739,922
- ^ Sneader W (2005). Drug Discovery: A History. New York: John Wiley & Sons. p. 68. ISBN 978-0-471-89979-2.
- ^ Jump up to:a b Simmons TJ, Lee SH, Park TJ, Hashim DP, Ajayan PM, Linhardt RJ (2009). “Antiseptic Single Wall Carbon Nanotube Bandages” (PDF). Carbon. 47 (6): 1561–1564. doi:10.1016/j.carbon.2009.02.005. Archived from the original (PDF) on 2010-06-21.
- ^ Simmons TJ, Hashim D, Vajtai R, Ajayan PM (August 2007). “Large area-aligned arrays from direct deposition of single-wall carbon nanotube inks”. Journal of the American Chemical Society. 129 (33): 10088–9. doi:10.1021/ja073745e. PMID 17663555.
Further reading
- Wong RH, Hung EC, Wong VW, Wan IY, Ng CS, Wan S, Underwood MJ (2009). “Povidone-iodine wound irrigation: A word of caution”. Surgical Practice. 13 (4): 123–4. doi:10.1111/j.1744-1633.2009.00461.x. S2CID 71797553.
- Wong RH, Wong VW, Hung EC, Lee PY, Ng CS, Wan IY, Underwood MJ (2011). “Topical application of povidone-iodine before wound closure is associated with significant increase in serum iodine level”. Surgical Practice. 19 (3): 79–82. doi:10.1111/j.1744-1633.2011.00547.x. S2CID 70528331.
- Wong RH, Ng CS, Underwood MJ (May 2012). “Iodine pleurodesis–a word of caution”. European Journal of Cardio-Thoracic Surgery. 41 (5): 1209. doi:10.1093/ejcts/ezr137. PMID 22219431.
External links
“Povidone-iodine”. Drug Information Portal. U.S. National Library of Medicine.
| Povidone-iodine applied to an abrasion using a cotton swab. | |
| Clinical data | |
|---|---|
| Trade names | Betadine, Wokadine, Pyodine, others |
| Other names | polyvidone iodine, iodopovidone |
| AHFS/Drugs.com | Consumer Drug Information |
| License data | US DailyMed: Povidone-iodine |
| Routes of administration | Topical |
| ATC code | D08AG02 (WHO)D09AA09 (WHO) (dressing)D11AC06 (WHO)G01AX11 (WHO)R02AA15 (WHO)S01AX18 (WHO)QG51AD01 (WHO) |
| Legal status | |
| Legal status | US: OTC / Rx-only |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 25655-41-8 |
| PubChem CID | 410087 |
| DrugBank | DB06812 |
| ChemSpider | none |
| UNII | 85H0HZU99M |
| KEGG | D00863C08043 |
| ChEBI | CHEBI:8347 |
| ChEMBL | ChEMBL1201724 |
| CompTox Dashboard (EPA) | DTXSID8035712 |
| ECHA InfoCard | 100.110.412 |
| Chemical and physical data | |
| Formula | (C6H9NO)n·xI |
| Molar mass | variable |
| (what is this?) (verify) |
///////////Povidone-iodine, PVP 1, BETADINE
C=CN1CCCC1=O.II
