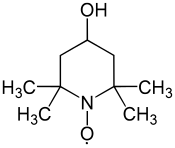
4-Hydroxy-TEMPO, TEMPOL, MBM-02, MTS 01
- Molecular FormulaC9H18NO2
- Average mass172.245 Da
2,2,6,6-Tetramethyl-4-hydroxypiperidinooxy
2,2,6,6-Tetramethyl-4-hydroxypiperidinooxy radical
2,2,6,6-Tetramethyl-4-piperidinol 1-oxyl
CAS 2226-96-2[RN]
4-hydroxy-1-oxyl-2,2,6,6-tetramethylpiperidine
4-Hydroxy-2,2,6,6-tetramethyl-1-piperidin-1-yloxy, free radical
4-Hydroxy-2,2,6,6-tetramethylpiperidine N-oxide
4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl
TEMPOLCAS Registry Number: 2226-96-2
CAS Name: 4-Hydroxy-2,2,6,6-tetramethyl-1-piperidinyloxy
Additional Names: 4-hydroxy-TEMPO; 4-hydroxy-2,2,6,6-tetramethyl piperidine N-oxide; 4-hydroxy-2,2,6,6-tetramethylpiperidinooxy
Molecular Formula: C9H18NO2, Molecular Weight: 172.24
Percent Composition: C 62.76%, H 10.53%, N 8.13%, O 18.58%
Literature References: Stable nitroxyl radical; water-soluble analogue of TEMPO, q.v. Functions as a membrane-permeable radical scavenger. Prepn: E. G. Rozantsev, Bull. Acad. Sci. USSR Div. Chem. Sci.12, 2085 (1964). Energy transfer studies: N. N. Quan, A. V. Guzzo, J. Phys. Chem.85, 140 (1981). IR conformation study: W. A. Bueno, L. Degrève, J. Mol. Struct.74, 291 (1981).Solid state NMR spectra: C. J. Groombridge, M. J. Perkins, J. Chem. Soc. Chem. Commun.1991, 1164. LC/MS/MS determn: I. D. Podmore, J. Chem. Res. Synop.2002, 574. Use as a phase transfer catalyst: X.-Y. Wang et al.,Synth. Commun.29, 157 (1999). Review of effects in animal models for shock, ischemia-reperfusion injury, and inflammation: C. Thiemermann, Crit. Care Med.31, S76-S84 (2003).
Properties: Crystals from ether + hexane, mp 71.5°. uv max (hexane): 240, 450-500 (e ~1800, ~5). uv max (ethanol): 242, 435-455 (e ~3800, ~10). Sol in water.
Melting point: mp 71.5°
Absorption maximum: uv max (hexane): 240, 450-500 (e ~1800, ~5); uv max (ethanol): 242, 435-455 (e ~3800, ~10)
Use: Spin label for EPR studies; phase transfer dehydration catalyst; antioxidant; inhibitor of olefin free radical polymerization.Topical PiperidineNitroxide MTS-01 is a topical gel containing a cell permeable hydrophilic piperidinenitroxide with potential radioprotective and antioxidant activity. As a stable, free radical compound, MTS-01 may be able to protect cells against the damaging effects of reactive oxygen species (ROS), upon exposure to ionizing radiation and oxidative stress. The topically applied MTS-01 may protect normal tissue from radiation-induced toxicity, such as radiation dermatitis, during radiation therapy.
4-Hydroxy-TEMPO is a member of aminoxyls and a member of piperidines. It has a role as a radical scavenger and a catalyst. It derives from a TEMPO.
4-Hydroxy-TEMPO or TEMPOL, formally 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, is a heterocyclic compound. Like the related TEMPO, it is used as a catalyst and chemical oxidant by virtue of being a stable aminoxyl radical. Its major appeal over TEMPO is that is less expensive, being produced from triacetone amine, which is itself made via the condensation of acetone and ammonia. This makes it economically viable on an industrial scale.[3]

Example synthesis of 4-Hydroxy-TEMPO from phorone, which is itself made from acetone and ammonia
In biochemical research, 4-hydroxy-TEMPO has been investigated as an agent for limiting reactive oxygen species. It catalyzes the disproportionation of superoxide, facilitates hydrogen peroxide metabolism, and inhibits Fenton chemistry.[4] 4-Hydroxy-TEMPO, along with related nitroxides, are being studied for their potential antioxidant properties.[5]
On an industrial-scale 4-hydroxy-TEMPO is often present as a structural element in hindered amine light stabilizers, which are commonly used stabilizers in plastics, it is also used as a polymerisation inhibitor, particularly during the purification of styrene.
It is a promising model substance to inhibit SARS-CoV-2 RNA-dependent RNA polymerase.[6]
SYN

SYN
Inorganica Chimica Acta, 370(1), 469-473; 2011
| IR | (KBr)vmax/cm-1: 3413 (m(O-H)) |
| Crystal Structure Data | Empirical formula C25H26NO8F6Cu; Formula weigh 646.02; T (K) 293(2); λ/Å 0.71073; Crystal system monoclinic; Space group P21/c; a (Å) 10.132(2); b (Å) 25.103(5); c (Å) 13.578(5); α (°) 90; β (°) 121.67(2); γ (°) 90; V (Å3) 2939.2(14); Z = 4 |
SYN
Bioorganic & Medicinal Chemistry Letters, 22(2), 920-923; 2012
SYN
https://pubs.acs.org/doi/10.1021/ol0712024
SYN
https://pubs.acs.org/doi/10.1021/es302157j
PAT
CN 113429392
SYN
Journal of the American Chemical Society, 138(29), 9069-9072; 2016
https://pubs.acs.org/doi/10.1021/jacs.6b05421
file:///C:/Users/Inspiron/Downloads/ja6b05421_si_001.pdf
| 1H NMR | (400 MHz, CDCl3) δH 3.89 (1H, tt, J = 11.4, 4.3 Hz, H4), 1.82.-.1.77 (2H, m, H3, H5), 1.43 (2H, t, J = 11.9 Hz, H3, H5), 1.14 (6H, s, 2 ×CH3), 1.07 (6H, s, 2 ×CH3); |
| 13C NMR | (100 MHz, CDCl3) δH 63.1, 47.5, 31.6, 20.6. |
| IR | (thin film, νmax / cm-1) 3407, 1472, 1376, 1174, 1161, 1066; |
| Rf | 0.22 (ethyl acetate / petroleum ether (1:1)); |

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com
////////////////////////////////////////
Thursday, June 3, 2021
NIH researchers identify potential new antiviral drug for COVID-19
Compound targets essential viral enzyme and prevents replication in cells.https://www.nih.gov/news-events/news-releases/nih-researchers-identify-potential-new-antiviral-drug-covid-19 small spherical structures in the center of the image are SARS-CoV-2 virus particles. The string-like protrusions extending from the cells are cell projections or pseudopodium. NIAID
small spherical structures in the center of the image are SARS-CoV-2 virus particles. The string-like protrusions extending from the cells are cell projections or pseudopodium. NIAID
The experimental drug TEMPOL may be a promising oral antiviral treatment for COVID-19, suggests a study of cell cultures by researchers at the National Institutes of Health. TEMPOL can limit SARS-CoV-2 infection by impairing the activity of a viral enzyme called RNA replicase. The work was led by researchers at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study appears in Science.
“We urgently need additional effective, accessible treatments for COVID-19,” said Diana W. Bianchi, M.D., NICHD Director. “An oral drug that prevents SARS-CoV-2 from replicating would be an important tool for reducing the severity of the disease.”
The study team was led by Tracey A. Rouault, M.D., head of the NICHD Section on Human Iron Metabolism. It discovered TEMPOL’s effectiveness by evaluating a more basic question on how the virus uses its RNA replicase, an enzyme that allows SARS-CoV-2 to replicate its genome and make copies of itself once inside a cell.
Researchers tested whether the RNA replicase (specifically the enzyme’s nsp12 subunit) requires iron-sulfur clusters for structural support. Their findings indicate that the SARS-CoV-2 RNA replicase requires two iron-sulfur clusters to function optimally. Earlier studies had mistakenly identified these iron-sulfur cluster binding sites for zinc-binding sites, likely because iron-sulfur clusters degrade easily under standard experimental conditions.
Identifying this characteristic of the RNA replicase also enables researchers to exploit a weakness in the virus. TEMPOL can degrade iron-sulfur clusters, and previous research from the Rouault Lab has shown the drug may be effective in other diseases that involve iron-sulfur clusters. In cell culture experiments with live SARS-CoV-2 virus, the study team found that the drug can inhibit viral replication.
Based on previous animal studies of TEMPOL in other diseases, the study authors noted that the TEMPOL doses used in their antiviral experiments could likely be achieved in tissues that are primary targets for the virus, such as the salivary glands and the lungs.
“Given TEMPOL’s safety profile and the dosage considered therapeutic in our study, we are hopeful,” said Dr. Rouault. “However, clinical studies are needed to determine if the drug is effective in patients, particularly early in the disease course when the virus begins to replicate.”
The study team plans on conducting additional animal studies and will seek opportunities to evaluate TEMPOL in a clinical study of COVID-19.
NIH authors on the study include researchers from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Institute of Neurological Disorders and Stroke. Authors from the Pennsylvania State University are funded by NIH’s National Institute of General Medical Sciences.
About the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): NICHD leads research and training to understand human development, improve reproductive health, enhance the lives of children and adolescents, and optimize abilities for all. For more information, visit https://www.nichd.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
Article
Maio N, et al. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science DOI: 10.1126/science.abi5224(link is external) (2021)
References
- ^ Zakrzewski, Jerzy; Krawczyk, Maria (1 January 2011). “Reactions of Nitroxides. Part XII [1]. – 2,2,6,6-Tetramethyl-1-oxyl- 4-piperidyl Chloroformate – A New Reactive Nitroxyl Radical. A One-pot Synthesis of 2,2,6,6-Tetramethyl-1-oxyl-4-piperidyl N,N-Dialkyl-carbamates”. Zeitschrift für Naturforschung B. 66 (5). doi:10.1515/znb-2011-0509.
- ^ Jump up to:a b c d Sigma-Aldrich Co., 4-Hydroxy-TEMPO. Retrieved on 2015-08-24.
- ^ Ciriminna, Rosaria; Pagliaro, Mario (15 January 2010). “Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives”. Organic Process Research & Development. 14 (1): 245–251. doi:10.1021/op900059x.
- ^ Wilcox, C. S.; Pearlman, A. (2008). “Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides”. Pharmacological Reviews. 60 (4): 418–69. doi:10.1124/pr.108.000240. PMC 2739999. PMID 19112152.
- ^ Lewandowski, M; Gwozdzinski, K. (2017). “Nitroxides as Antioxidants and Anticancer Drugs”. International Journal of Molecular Sciences. 18 (11): 2490. doi:10.3390/ijms18112490. PMC 5713456.
- ^ Maio, N.; Lafont, B.A.P.; Sil, D.; Li, Y.; Bollinger, M.; Krebs, C. (2021). “Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets”. Science. 373 (6551): 236–241. doi:10.1126/science.abi5224.
| Names | |
|---|---|
| Preferred IUPAC name(4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)oxyl | |
| Other namestempol; tanol; TMPN; 4-Oxypiperidol; nitroxyl 2; HyTEMPO | |
| Identifiers | |
| CAS Number | 2226-96-2 |
| 3D model (JSmol) | Interactive image |
| ChEBI | CHEBI:180664 |
| ChEMBL | ChEMBL607023 |
| ChemSpider | 121639 |
| ECHA InfoCard | 100.017.056 |
| PubChem CID | 137994 |
| UNII | U78ZX2F65X |
| CompTox Dashboard (EPA) | DTXSID4041280 |
| showInChI | |
| showSMILES | |
| Properties | |
| Chemical formula | C9H18NO2 |
| Molar mass | 172.248 g·mol−1 |
| Appearance | Orange crystals |
| Melting point | 71–73 °C (160–163 °F; 344–346 K)[1] |
| Solubility in water | 629.3 g/l (20 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms | [2] |
| Signal word | Warning[2] |
| Hazard statements | H302, H315, H319, H335[2] |
| Precautionary statements | P261, P305+P351+P338[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
 verify (what is ?) verify (what is ?) | |
| Infobox references |
//////////////////4-Hydroxy-TEMPO, TEMPOL, MBM-02, MTS 01, ZJ 701, CORONA VIRUS, COVID 19
https://www.clinicaltrials.gov/ct2/show/NCT04729595
CC1(CC(CC(N1[O])(C)C)O)C
