PILOCARPINE
- Molecular FormulaC11H16N2O2
- Average mass208.257 Da
2(3H)-Furanone, 3-ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl]-, (3S-cis)-
202-128-4[EINECS]92-13-7 CAS
54-71-7[RN]
(+)-pilocarpine
(3S,4R)-3-Ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]dihydro-2(3H)-furanone
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Pilocarpine hydrochloride | 0WW6D218XJ | 54-71-7 | RNAICSBVACLLGM-GNAZCLTHSA-N |
| Pilocarpine nitrate | M20T465H6J | 148-72-1 | PRZXEPJJHQYOGF-GNAZCLTHSA-N |
PilocarpineCAS Registry Number: 92-13-7
CAS Name: (3S-cis)-3-Ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl]-2(3H)-furanone
Trademarks: Ocusert Pilo (Cusi)
Molecular Formula: C11H16N2O2, Molecular Weight: 208.26
Percent Composition: C 63.44%, H 7.74%, N 13.45%, O 15.36%
Literature References: Cholinergic principle from Pilocarpus jaborandi Holmes, Rutaceae. Isoln: Petit, Polanovski, Bull. Soc. Chim. [3] 17, 557, 702 (1897). Structure: Jowett, J. Chem. Soc.77, 473, 851 (1900); 83, 438 (1903). Stereoisomeric with isopilocarpine: Polonovski, Polonovski, Bull. Soc. Chim. [4] 31, 1314 (1922). Has the cis configuration; isopilocarpine is trans: Zav’yalov, Dokl. Akad. Nauk SSSR82, 257 (1952). Absolute configuration: Hill, Barcza, Tetrahedron22, 2889 (1966). Synthesis: Preobrashenski et al.,Ber.66, 1187 (1933); Samokhvalov, Med. Prom. SSSR11, no. 2, 10 (1957); DeGraw, Tetrahedron28, 967 (1972); Link, Bernauer, Helv. Chim. Acta55, 1053 (1972). Stereoselective synthesis: A. Noordam et al.,Rec. Trav. Chim.98, 467 (1979). Review: Langenbeck, Angew. Chem.60, 297 (1948); van Rossum et al.,Experientia16, 373 (1960). Toxicity studies: Beccari, Boll. Chim. Farm.106, 8 (1967). Comprehensive description: A. A. Al-Badr, H. Y. Aboul-Enein, Anal. Profiles Drug Subs.12, 385-432 (1983). Clinical trial in Sjögren’s syndrome: F. B. Vivino et al., Arch. Intern. Med.159, 174 (1999); in radiation-induced xerostomia: J.-C. Horiot et al.,Radiother. Oncol.55, 233 (2000).
Properties: Oil or crystals, mp 34°. bp5 260° (partial conversion to isopilocarpine). [a]D18 +106° (c = 2). pK1 (20°) 7.15; pK2 (20°) 12.57. Sol in water, alcohol, chloroform; sparingly sol in ether, benzene. Almost insol in petr ether.
Melting point: mp 34°
Boiling point: bp5 260° (partial conversion to isopilocarpine)
pKa: pK1 (20°) 7.15; pK2 (20°) 12.57
Optical Rotation: [a]D18 +106° (c = 2)
Derivative Type: Hydrochloride
CAS Registry Number: 54-71-7
Trademarks: Akarpine (Akorn); Almocarpine (Ayerst); Isopto Carpine (Alcon); Pilogel (Alcon); Pilopine HS (Alcon); Pilostat (Bausch & Lomb); Salagen (MGI)
Molecular Formula: C11H16N2O2.HCl, Molecular Weight: 244.72
Percent Composition: C 53.99%, H 7.00%, N 11.45%, O 13.08%, Cl 14.49%
Properties: Hygroscopic crystals from alcohol, mp 204-205°. [a]D18 +91° (c = 2). Freely sol in water, alcohol. Practically insol in ether, chloroform. Keep well closed and protected from light.
Melting point: mp 204-205°
Optical Rotation: [a]D18 +91° (c = 2)
Derivative Type: Nitrate
CAS Registry Number: 148-72-1
Trademarks: Chibro Pilocarpine (Chibret); Licarpin (Allergan); Pilo (Novopharma); Pilofrin (Allergan); Pilagan (Allergan)
Molecular Formula: C11H16N2O2.HNO3, Molecular Weight: 271.27
Percent Composition: C 48.70%, H 6.32%, N 15.49%, O 29.49%
Properties: mp 173.5-174.0° (dec). Poisonous! [a]D +77 to +83° (c = 10). One gram dissolves in 4 ml water, 75 ml alcohol. Insol in chloroform, ether. Incompat. Silver nitrate, mercury bichloride, iodides, gold salts, tannin, calomel, KMnO4, alkalies.
Melting point: mp 173.5-174.0° (dec)
Optical Rotation: [a]D +77 to +83° (c = 10)
Derivative Type: Isopilocarpine
Additional Names: b-Pilocarpine
Properties: Hygroscopic oily liquid or prisms. bp10 261°. [a]D18 +50° (c = 2). pK1 (18°) 7.17. Miscible with water and alcohol; very sol in chloroform; less sol in benzene, ether. Almost insol in petr ether.
Boiling point: bp10 261°
pKa: pK1 (18°) 7.17
Optical Rotation: [a]D18 +50° (c = 2)
Derivative Type: Isopilocarpine hydrochloride hemihydrate
Molecular Formula: C11H16N2O2.HCl.½H2O, Molecular Weight: 253.73
Percent Composition: C 52.07%, H 7.15%, N 11.04%, O 15.76%, Cl 13.97%
Properties: Scales from alcohol + ether, mp 127°; when anhydr, mp 161°. [a]D18 +39° (c = 5). Sol in 0.27 part water; 2.1 parts alcohol.
Melting point: mp 127°; mp 161°
Optical Rotation: [a]D18 +39° (c = 5)
Derivative Type: Isopilocarpine nitrate
Molecular Formula: C11H16N2O2.HNO3, Molecular Weight: 271.27Percent Composition: C 48.70%, H 6.32%, N 15.49%, O 29.49%
Properties: Prisms from water, scales from alcohol, mp 159°. [a]D18 +39° (c = 2). Sol in 8.4 parts water, in 350 parts abs alcohol.
Melting point: mp 159°
Optical Rotation: [a]D18 +39° (c = 2)
Therap-Cat: Antiglaucoma agent; miotic; sialogogue.
Therap-Cat-Vet: Parasympathomimetic; miotic; gastric secretory stimulant.
Keywords: Antiglaucoma; Miotic; Sialagogue.
Pilocarpine is a muscarinic cholinergic agonist used on the eye to treat elevated intraocular pressure, various types of glaucoma, and to induce miosis. Also available orally to treat symptoms of dry mouth associated with Sjogren’s syndrome and radiotherapy.
Pilocarpine is a medication used to reduce pressure inside the eye and treat dry mouth.[1][3] As eye drops it is used to manage angle closure glaucoma until surgery can be performed, ocular hypertension, primary open angle glaucoma, and to bring about constriction of the pupil following its dilation.[1][4][5] However, due to its side effects it is no longer typically used in the long term management.[6] Onset of effects with the drops is typically within an hour and lasts for up to a day.[1] By mouth it is used for dry mouth as a result of Sjögren syndrome or radiation therapy.[7]
Common side effects of the eye drops include irritation of the eye, increased tearing, headache, and blurry vision.[1] Other side effects include allergic reactions and retinal detachment.[1] Use is generally not recommended during pregnancy.[8] Pilocarpine is in the miotics family of medication.[9] It works by activating cholinergic receptors of the muscarinic type which cause the trabecular meshwork to open and the aqueous humor to drain from the eye.[1]
Pilocarpine was isolated in 1874 by Hardy and Gerrard and has been used to treat glaucoma for more than 100 years.[10][11][12] It is on the World Health Organization’s List of Essential Medicines.[13] It was originally made from the South American plant Pilocarpus.[10]

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com
////////////////////////////////////////
Pilocarpine hydrochloride, KSS-694, MGI-647, Pilobuc, Pilocar, Isopto carpine, Spersacarpin, Pilo, Isopto-pilocarpine, Pilocarpina lux, Pilogel, PilaSite(sustained release), Salagen, Pilopine HS
SYN
The alkylation of pilosine (I) with ethyl chloride (II) by means of LDA in THF gives trans-pilocarpine (III), which is isomerized with LDA in THF, yielding a mixture of cis- and trans-pilocarpine (IV). Finally, this mixture is resolved by crystallization with di-p-toluoyl tartaric acid.
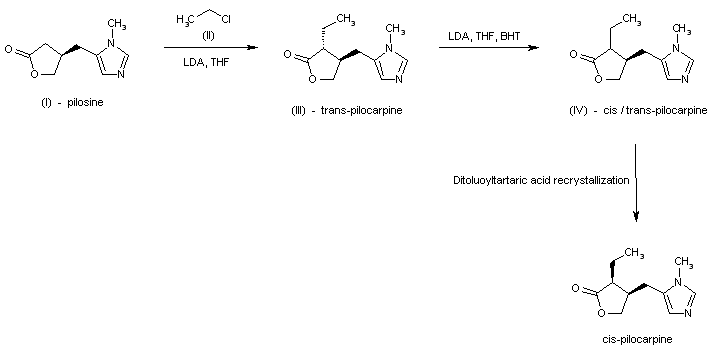
SYN
Journal of Organic Chemistry, 58(1), 62-4; 1993
https://pubs.acs.org/doi/abs/10.1021/jo00053a016
SYN
Tetrahedron, 65(39), 8283-8296; 2009
SYN
Science of Synthesis, 20b, 987-1046; 2006
SYN
https://linkinghub.elsevier.com/retrieve/pii/S0040402008014002


SYN
https://www.mdpi.com/1420-3049/26/12/3676/htm
Schmidt, Theresa et alFrom Molecules, 26(12), 3676; 2021
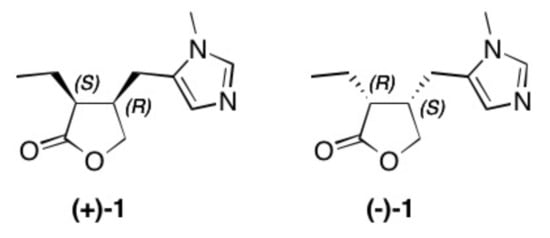
Figure 1. Structure of natural occurring pilocarpine (+)-1 and its enantiomer (–)-1.
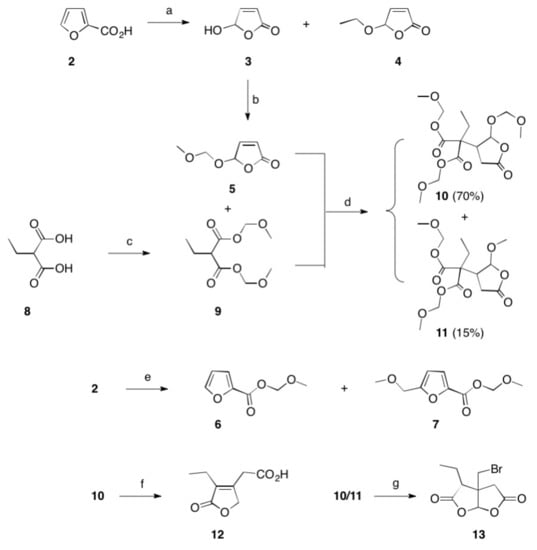
Scheme 1. Reactions and conditions: (a) hν, Bengal rosa, 8 h, 20 °C, 76% (of 3) and 5% (of 4); (b) CH2(OCH3)2, P4O10, DCM, 20 °C, 5 h, 98%; (c) CH2(OCH3)2, P4O10, DCM, 20 °C, 5 h, 99%; (d) THF, Na, 25 °C, 15 h, 72%; (e) CH2(OCH3)2, P4O10, DCM, 20 °C, 5 h, 77% (of 6) and 19% (of 7); (f) HBr, reflux, 2 d, 83%; (g) HBr, reflux, 4 d, 4%.
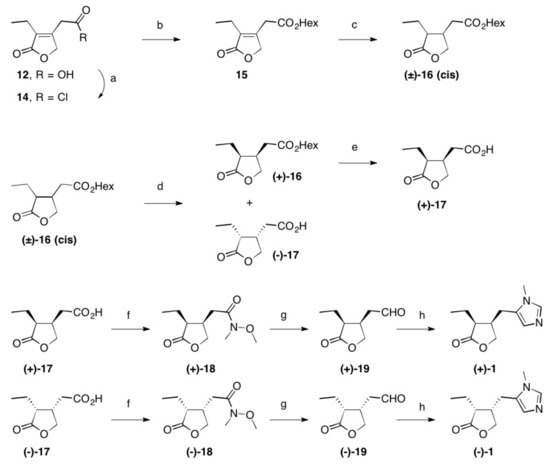
Scheme 2. Reactions and conditions: (a) SOCl2, reflux, 3 h, quant.; (b) Hex-OH, reflux, 16 h, 98%; (c) Rh/Al2O3, H2 (1 at), THF, 5 d, quant.; (d) Lipase PS, pH = 7.0, 2 d, 22 °C, 48% (of (±)-16) and 42% (of (–)-17); (e) PLE, pH = 7.0, 22 °C, 2 d, 96%; (f) N-methylmorpholine, iBu-chloroformate, N,O-dimethylhydroxylamine hydrochloride, 23 °C, 1 d, 84% (of (+)-18) and 85% of (–)-18); (g) LiAlH4, Et2O, 23 °C, 30 min, 95% (of (+)-19) and 95% of (–)-19; (h) CH3NH2, TosMic, DCM, benzene, NEt3, 7 d, 23 °C, 59% (of (+1)-1 and 60% of (–)-1; Hex stands for n-hexyl.
(+)-Pilocarpine [(+)-1]
Following the procedure given for the synthesis of its enantiomer, (+)-1 (1.92 g, 59%) was obtained as a colorless oil; Rf = 0.60 (SiO2, DCM/MeOH/aq NH4OH (25%), 95:4:1); [α]D = +115.7° (c 0.6, CHCl3), ee > 99% (by HPLC, Chiralcel OC, n-hexane/ethanol, 3:7, 0.3 mL/min, UV-detection λ = 215 nm; tR = (+)-1 47.1 min, tR = (–)-1 = 52.32 min); IR (film), 1H-NMR, 13C-NMR and MS (ESI, MeOH) were identical to the enantiomer (vide supra); analysis calcd. for C11H16N2O2 (208.26): C 63.44, H 7.74, N 13.45; found: C 63.31, H 7.98, N 13.32
PAPERBy Fuerstner, AloisFrom e-EROS Encyclopedia of Reagents for Organic Synthesis, 1-7; 2001
| Clinical data | |
|---|---|
| Trade names | Isopto Carpine, Salagen, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608039 |
| Pregnancy category | AU: B3 |
| Routes of administration | Topical eye drops, by mouth |
| Drug class | Miotic (cholinergic)[1] |
| ATC code | N07AX01 (WHO) S01EB01 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)UK: POM (Prescription only)US: ℞-only |
| Pharmacokinetic data | |
| Elimination half-life | 0.76 hours (5 mg), 1.35 hours (10 mg)[2] |
| Excretion | urine |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 92-13-7 54-71-7 (hydrochloride) |
| PubChem CID | 5910 |
| IUPHAR/BPS | 305 |
| DrugBank | DB01085 |
| ChemSpider | 5699 |
| UNII | 01MI4Q9DI3 |
| KEGG | D00525 |
| ChEBI | CHEBI:8207 |
| ChEMBL | ChEMBL550 |
| CompTox Dashboard (EPA) | DTXSID1021162 |
| ECHA InfoCard | 100.001.936 |
| Chemical and physical data | |
| Formula | C11H16N2O2 |
| Molar mass | 208.261 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (verify) |
Medical uses
Pilocarpine stimulates the secretion of large amounts of saliva and sweat.[14] It is used to prevent or treat dry mouth, particularly in Sjögren syndrome, but also as a side effect of radiation therapy for head and neck cancer.[15]
It may be used to help differentiate Adie syndrome from other causes of unequal pupil size.[16][17][clarification needed]
It may be used to treat a form of dry eye called aqueous deficient dry eye (ADDE)[18]
Surgery
Pilocarpine is sometimes used immediately before certain types of corneal grafts and cataract surgery.[19][20] In ophthalmology, pilocarpine is also used to reduce symptomatic glare at night from lights when the patient has undergone implantation of phakic intraocular lenses; the use of pilocarpine would reduce the size of the pupils, partially relieving these symptoms.[dubious – discuss] The most common concentration for this use is pilocarpine 1%.[citation needed] Pilocarpine is shown to be just as effective as apraclonidine in preventing intraocular pressure spikes after laser trabeculoplasty.[21]
Presbyopia
In 2021, the US Food and Drug Administration approved pilocarpine hydrochloride as an eyedrop treatment for presbyopia, age-related difficulty with near-in vision. Marketed as vuity, the effect lasts for 7 to 10 hours.[22]
Other
Pilocarpine is used to stimulate sweat glands in a sweat test to measure the concentration of chloride and sodium that is excreted in sweat. It is used to diagnose cystic fibrosis.[23]
Adverse effects
Use of pilocarpine may result in a range of adverse effects, most of them related to its non-selective action as a muscarinic receptor agonist. Pilocarpine has been known to cause excessive salivation, sweating, bronchial mucus secretion, bronchospasm, bradycardia, vasodilation, and diarrhea. Eye drops can result in brow ache and chronic use in miosis.
Pharmacology
Pilocarpine is a drug that acts as a muscarinic receptor agonist. It acts on a subtype of muscarinic receptor (M3) found on the iris sphincter muscle, causing the muscle to contract – resulting in pupil constriction (miosis). Pilocarpine also acts on the ciliary muscle and causes it to contract. When the ciliary muscle contracts, it opens the trabecular meshwork through increased tension on the scleral spur. This action facilitates the rate that aqueous humor leaves the eye to decrease intraocular pressure. Paradoxically, when pilocarpine induces this ciliary muscle contraction (known as an accommodative spasm) it causes the eye’s lens to thicken and move forward within the eye. This movement causes the iris (which is located immediately in front of the lens) to also move forward, narrowing the Anterior chamber angle. Narrowing of the anterior chamber angle increases the risk of increased intraocular pressure.[24]
Society and culture
Preparation
Plants in the genus Pilocarpus are the only known sources of pilocarpine, and commercial production is derived entirely from the leaves of Pilocarpus microphyllus (Maranham Jaborandi). This genus grows only in South America, and Pilocarpus microphyllus is native to several states in northern Brazil.[25]
Pilocarpine is extracted from the powdered leaf material in a multi-step process. First the material is treated with ethanol acidified with hydrochloric acid, and the solvents removed under reduced pressure. The resultant aqueous residue is neutralized with ammonia and put aside until the resin has completely settled. It is then filtered and concentrated by sugar solution to a small volume, made alkaline with ammonia, and finally extracted with chloroform. The solvent is removed under reduced pressure.[verification needed]
Cost
Pilocarpine is one of the lowest cost medications for glaucoma.[26]
Trade names
Pilocarpine is available under several trade names such as: Diocarpine (Dioptic), Isopto Carpine (Alcon), Miocarpine (CIBA Vision), Ocusert Pilo-20 and -40 (Alza), Pilopine HS (Alcon), Salagen (MGI Pharma), Scheinpharm Pilocarpine (Schein Pharmaceutical), Timpilo (Merck Frosst) and Vuity (Abbvie).
Research
Pilocarpine is used to induce chronic epilepsy in rodents, commonly rats, as a means to study the disorder’s physiology and to examine different treatments.[27][28] Smaller doses may be used to induce salivation in order to collect samples of saliva, for instance, to obtain information about IgA antibodies.
Veterinary
Pilocarpine is given in moderate doses (about 2 mg) to induce emesis in cats that have ingested foreign plants, foods, or drugs. One feline trial determined it was effective, even though the usual choice of emetic is xylazine.
References
- ^ Jump up to:a b c d e f g “Pilocarpine”. The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- ^ Gornitsky M, Shenouda G, Sultanem K, Katz H, Hier M, Black M, Velly AM (July 2004). “Double-blind randomized, placebo-controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer”. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 98 (1): 45–52. doi:10.1016/j.tripleo.2004.04.009. PMID 15243470.
- ^ Tarascon Pocket Pharmacopoeia 2019 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2018. p. 224. ISBN 9781284167542.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 439. hdl:10665/44053. ISBN 9789241547659.
- ^ “Glaucoma and ocular hypertension. NICE guideline 81”. National Institute for Health and Care Excellence. November 2017. Retrieved 19 September 2019.
Ocular hypertension… alternative options include carbonic anhydrase inhibitors such as brinzolamide or dorzolamide, a topical sympathomimetic such as apraclonidine or brimonidine tartrate, or a topical miotic such as pilocarpine, given either as monotherapy or as combination therapy.
- ^ Lusthaus J, Goldberg I (March 2019). “Current management of glaucoma” (PDF). The Medical Journal of Australia. 210 (4): 180–187. doi:10.5694/mja2.50020. PMID 30767238. S2CID 73438590.
Pilocarpine is no longer routinely used for long term IOP control due to a poor side effect profile
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 415. ISBN 9781284057560.
- ^ “Pilocarpine ophthalmic Use During Pregnancy | Drugs.com”. http://www.drugs.com. Archived from the original on 28 December 2016. Retrieved 28 December 2016.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 769. ISBN 9780857111562.
- ^ Jump up to:a b Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 98. ISBN 978-0-471-89979-2. Archived from the original on 2016-12-29.
- ^ Rosin A (1991). “[Pilocarpine. A miotic of choice in the treatment of glaucoma has passed 110 years of use]”. Oftalmologia (in Romanian). 35 (1): 53–5. PMID 1811739.
- ^ Holmstedt, B; Wassén, SH; Schultes, RE (January 1979). “Jaborandi: an interdisciplinary appraisal”. Journal of Ethnopharmacology. 1 (1): 3–21. doi:10.1016/0378-8741(79)90014-x. PMID 397371.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ “Pilocarpine”. MedLinePlus. U.S. National Library of Medicine. Archived from the original on 2010-03-06.
- ^ Yang, WF; Liao, GQ; Hakim, SG; Ouyang, DQ; Ringash, J; Su, YX (1 March 2016). “Is Pilocarpine Effective in Preventing Radiation-Induced Xerostomia? A Systematic Review and Meta-analysis”. International Journal of Radiation Oncology, Biology, Physics. 94 (3): 503–11. doi:10.1016/j.ijrobp.2015.11.012. hdl:10722/229069. PMID 26867879.
- ^ Kanski JJ, Bowling B (2015-03-24). Kanski’s Clinical Ophthalmology E-Book: A Systematic Approach. Elsevier Health Sciences. p. 812. ISBN 9780702055744.
- ^ Bartlett JD, James SD (October 2013). “Drug Affect the Autonomous Nervous System”. Clinical Ocular Pharmacology. Elsevier. p. 118. ISBN 9781483193915.
- ^ Mannis, Mark J; Holland, Edward J (September 2016). “Chapter 33: Dry Eye”. Cornea E-Book. Elsevier Health Sciences. p. 388. ISBN 978-0-323-35758-6. OCLC 960165358.
- ^ Parker, Jack (2017). Descemet Membrane Endothelial Keratoplasty (DMEK): A Review (PDF) (Thesis). Leiden University.
- ^ Ahmed E, E A (2010). Comprehensive Manual of Ophthalmology. JP Medical Ltd. p. 345. ISBN 9789350251751.
- ^ Zhang L, Weizer JS, Musch DC (February 2017). “Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty”. The Cochrane Database of Systematic Reviews. 2 (2): CD010746. doi:10.1002/14651858.CD010746.pub2. PMC 5477062. PMID 28231380.
- ^ Bankhead, Charles (2021-11-01). “First Eye Drop Treatment for Presbyopia Wins FDA Approval”. http://www.medpagetoday.com. Retrieved 2021-11-02.
- ^ Prasad RK (2017-07-11). Chemistry and Synthesis of Medicinal Agents: (Expanding Knowledge of Drug Chemistry). BookRix. ISBN 9783743821415.
- ^ Shaarawy TM, Sherwood MB, Hitchings RA, Crowston JG (September 2014). “Lsser Peripheral Iridoplasty”. Glaucoma E-Book. Elsevier Health Sciences. p. 718. ISBN 9780702055416.
- ^ De Abreu IN, Sawaya AC, Eberlin MN, Mazzafera P (November–December 2005). “Production of Pilocarpine in Callus of Jaborandi (Pilocarpus microphyllus Stapf)”. In Vitro Cellular & Developmental Biology – Plant. Society for In Vitro Biology. 41 (6): 806–811. doi:10.1079/IVP2005711. JSTOR 4293939. S2CID 26058596.
- ^ Schwab, Larry (2007). Eye Care in Developing Nations. CRC Press. p. 110. ISBN 9781840765229.
- ^ Károly N (2018). Immunohistochemical investigations of the neuronal changes induced by chronic recurrent seizures in a pilocarpine rodent model of temporal lobe epilepsy (Thesis). University of Szeged. doi:10.14232/phd.9734.
- ^ Morimoto K, Fahnestock M, Racine RJ (May 2004). “Kindling and status epilepticus models of epilepsy: rewiring the brain”. Progress in Neurobiology. 73 (1): 1–60. doi:10.1016/j.pneurobio.2004.03.009. PMID 15193778. S2CID 36849482.
External links
- “Pilocarpine”. Drug Information Portal. U.S. National Library of Medicine.
CLIP
Firms Team Up To Sustain Natural Pilocarpine
Sustainable harvest is key to a new pharmaceutical chemicals venture
https://cen.acs.org/articles/93/i11/Firms-Team-Sustain-Natural-Pilocarpine.html
Last summer, Andrew Badrot bought a portfolio of plant-sourced pharmaceutical chemicals from Boehringer Ingelheim and acquired BI’s distribution rights for pilocarpine, a plant-derived glaucoma treatment.
For BI, the transactions were small ones. The German drugmaker had been exiting its private-label active pharmaceutical ingredients (API) business, scaling back to produce only the chemicals it uses to manufacture its own drugs.
But for Badrot the deals were potentially big. He leads the company that bought the businesses—Centroflora CMS, a joint venture between the Brazilian botanicals firm Centroflora and CMS Pharma, Badrot’s custom chemicals consultancy. Together, Centroflora and Centroflora CMS are committed to nurturing the natural source of pilocarpine, an alkaloid used medicinally for more than 100 years, and to expanding into other APIs neglected by larger firms.
Pilocarpine’s source, Pilocarpus microphyllus, better known as jaborandi, had been harvested vigorously in the wild by Merck KGaA, which in 1975 built a factory in Parnaíba in northern Brazil to extract pilocarpine. By the mid-1980s, however, jaborandi had been overharvested, and the government declared it a protected species. Merck began obtaining the leaves from a plantation in the northern Brazilian state of Maranhão.
Demand for the drug as a glaucoma treatment began to decline, and Merck eventually closed the plant. When the market for the drug revived with new indications as a dry-mouth remedy, the company saw an opportunity to sell the site and did so in 2002.
The buyer was Centroflora, which was founded in 1957 in São Paulo. The firm was interested in adding pilocarpine to its botanical extracts business, according to its chief executive, Peter Andersen, a native of Brazil whose coffee-trader father bought into Centroflora in 1983. Along with the purchase, Centroflora signed a deal for BI to distribute the drug.
The company wanted to revitalize natural harvesting of jaborandi and began working with the Brazilian government to promulgate sustainable practices in the field. Centroflora also worked closely with a German government agency, Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ), which promotes sustainable harvesting internationally and had been working in the north of Brazil for decades.
Centroflora’s distribution agreement with BI arose through connections at GIZ, according to Andersen. BI also had been Merck’s biggest customer for pilocarpine.
But ecological sustainability was only half of the problem, Andersen says. Centroflora also found itself dealing with middlemen who would collect the jaborandi from poor family farms in remote areas and pay them next to nothing. Establishing a direct supply channel was not easy.

“I can spend a few days telling you about that process,” he says. “Stories of difficult relationships and difficult moments. But in some cases we managed to hire some of the middlemen to work for us on a salary basis. They made less money, but they had a job.”
Today, farmers in Brazil are paid at least twice what they were paid by intermediaries, Andersen says.
Key to the process was a program Centroflora launched in 2004 called Partnerships for a Better World to train and certify growers, establish community associations to support growers, and maintain sustainable harvesting practices.
Centroflora is the leading supplier of pilocarpine. Its only competitor, Sourcetech, with a plant near São Paulo, accesses jaborandi from the plantation that supplied Merck, now owned by U.S.-based Quercegen.
Pilocarpine accounts for only about 5% of Centroflora’s $95 million in annual sales. The company produces a long list of botanical extracts, including nutritional supplements and herbal medicines such as acai, acerola, coffee powder, and powdered fruit.The company manufactures at four facilities in Brazil, including the former Merck plant, which is dedicated to pilocarpine. But Andersen sees the partnership with CMS as a route to increase phytochemical API manufacturing at that site.
“The facility has the capacity to produce 12 metric tons per year of alkaloids,” Andersen says. It currently makes less than three metric tons. “So there is a lot of space to produce more, and the idea is that we can do some of the APIs we got from Boehringer Ingelheim.”
Those include atropine, digoxin, homatropine, and dihydroergotamine mesylate. Centroflora CMS also obtained distribution rights to BI’s scopolamine N-butyl bromide. All are derived from botanicals harvested on farms around the world.
Badrot was vice president of strategy for Lonza’s exclusive synthesis division before starting CMS in 2010 to consult on manufacturing and mergers and acquisitions in the custom chemicals business. “But for me, the dream was to return to manufacturing APIs,” he says.
The phytochemicals portfolio, including some of the oldest APIs made by BI, for which CMS has done consulting work, seemed like an ideal reentry to manufacturing, according to Badrot. “They are niche products that maybe fly a bit under the radar,” he says. “They seemed to fit us well because we can give them some attention.”
Centroflora CMS’s first order of business, he says, is to establish manufacturing for the BI products, which BI will continue to make until then. Badrot says Centroflora is well suited to manufacture at least the digoxin and atropine, but decisions have not been finalized. The partners will likely use contract manufacturers for some of the products. And Badrot says Centroflora CMS seeks to replicate the kind of deal it has with BI.
“We are looking for other companies with APIs that represent 0–1% of sales, products that lack focus,” he says. “We would take them over.”
Badrot and Andersen say they are also interested in sharing the Partnerships for a Better World program with other companies involved in harvesting natural products. And Centroflora looks for other ways to support its supply chain. Last month, it was approved as a trading member of the Union for Ethical BioTrade, a nonprofit that promotes sustainable development and biodiversity. As a member, Centroflora commits to sustainable sourcing practices and will be required to undergo periodic audits.
Last year, Centroflora received government recognition for its efforts on both the environmental and social fronts. The National Confederation of Industry in Brazil named Centroflora’s jaborandi harvesting program one of the country’s 10 most sustainable business practices. And Banco do Brasil, the national bank, recognized the firm for its work to improve conditions for farmers in the northern forest region of the country.
As the joint venture starts to work with its new portfolio of phytochemicals, both Andersen and Badrot look back at the jaborandi success as the road forward, a template for fostering a plant-based API business that may inspire other companies.
For Andersen, Partnerships for a Better World is an essential foundation of trust for the ecological and socially responsible harvesting of botanicals in Brazil. “There were a lot of problems along the way,” he says. “But we are at peace with it today.”
////////////////PILOCARPINE, Pilocarpine hydrochloride, KSS-694, MGI-647, Pilobuc, Pilocar, Isopto carpine, Spersacarpin, Pilo, Isopto-pilocarpine, Pilocarpina lux, Pilogel, PilaSite(sustained release), Salagen, Pilopine HS
CC[C@H]1[C@@H](CC2=CN=CN2C)COC1=O
