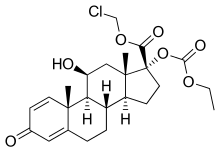

loteprednol etabonate
- Molecular FormulaC24H31ClO7
- Average mass466.952 Da
cas 82034-46-6
chloromethyl (8S,9S,10R,11S,13S,14S,17R)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-17-carboxylate(11b,17a)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-androsta-1,4-diene-17-carboxylic acid chloromethyl ester
(11b,17a)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylic Acid Chloromethyl Ester
(8S,9S,10R,11S,13S,14S,17R)-17-[(éthoxycarbonyl)oxy]-11-hydroxy-10,13-diméthyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrène-17-carboxylate de chlorométhyle
129260-79-3[RN]
17a-Ethoxycarbonyloxy-D’-cortienic Acid Chloromethyl Ester
82034-46-6[RN]
Androsta-1,4-diene-17-carboxylic acid, 17-((ethoxycarbonyl)oxy)-11-hydroxy-3-oxo-, chloromethyl ester, (11β,17α)-
Androsta-1,4-diene-17-carboxylic acid, 17-[(ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-, chloromethyl ester, (11β,17α)-
Loteprednol Etabonate
CAS Registry Number: 82034-46-6
CAS Name: (11b,17a)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylic acid chloromethyl ester
Additional Names: chloromethyl 17a-ethoxycarbonyloxy-11b-hydroxyandrosta-1,4-diene-3-one-17b-carboxylate; 17a-ethoxycarbonyloxy-D¢-cortienic acid chloromethyl ester
Manufacturers’ Codes: CDDD-5604; HGP-1; P-5604
Trademarks: Alrex (Bausch & Lomb); Lotemax (Bausch & Lomb)
Molecular Formula: C24H31ClO7, Molecular Weight: 466.95
Percent Composition: C 61.73%, H 6.69%, Cl 7.59%, O 23.98%
Literature References: Ophthalmic corticosteroid. Prepn: N. S. Bodor, BE889563 (1981 to Otsuka); idem,US4996335 (1991). Physicochemical properties: M. Alberth et al.,J. Biopharm. Sci.2, 115 (1991). HPLC determn in plasma and urine: G. Hochhaus et al.,J. Pharm. Sci.81, 1210 (1992). NMR structural studies: S. Rachwal et al.,Steroids61, 524 (1996); idem et al., ibid. 63, 193 (1998). Metabolism and transdermal permeability: N. Bodor et al.,Pharm. Res.9, 1275 (1992). Evaluation of effect on intraocular pressure: J. D. Bartlett et al.,J. Ocul. Pharmacol.9, 157 (1993). Clinical trial in keratoconjunctivitis sicca: S. C. Pflugfelder et al.,Am. J. Ophthalmol.138, 444 (2004). Review of ophthalmic clinical studies: J. F. Howes, Pharmazie55, 178-183 (2000).
Properties: Crystals from THF + hexane, mp 220.5-223.5°. Soly at 25° (mg/ml): 0.0005 in water; 0.037 in 50% propylene glycol + water. Lipophilicity (log K): 3.04.
Melting point: mp 220.5-223.5°
Therap-Cat: Anti-inflammatory (topical).
Keywords: Glucocorticoid.
Research Code:HGP-1; CDDD-5604; P-5604Trade Name:Lotemax® / Alrex®MOA:CorticosteroidIndication:Acne rosacea; Superficial punctate keratitis; Postoperative inflammation and pain following ocular surgery; Iritis; Herpes zoster keratitis; Allergic conjunctivitis; CyclitisCompany:Bausch & Lomb (Originator)Sales:ATC Code:S01BA14
Loteprednol etabonate was approved by the U.S. Food and Drug Administration (FDA) on Mar 9, 1998. It was developed and marketed as Lotemax® by Bausch & Lomb.
Loteprednol etabonate is a corticosteroid used in ophthalmology. It is indicated for the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe such as allergic conjunctivitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, selected infective conjunctivitides.
Lotemax® is available as drops for ophthalmic use, containing 0.5% of Loteprednol etabonate. The recommended dose is one to two drops into the conjunctival sac of the affected eyes four times daily.
Loteprednol (as the ester loteprednol etabonate) is a corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax[1] and Loterex.
It was patented in 1980 and approved for medical use in 1998.[2]
Loteprednol Etabonate is the etabonate salt form of loteprednol, an ophthalmic analog of the corticosteroid prednisolone with anti-inflammatory activity. Loteprednol etabonate exerts its effect by interacting with specific intracellular receptors and subsequently binds to DNA to modify gene expression. This results in an induction of the synthesis of certain anti-inflammatory proteins while inhibiting the synthesis of certain inflammatory mediators. Loteprednol etabonate specifically induces phospholipase A2 inhibitory proteins (collectively called lipocortins), which inhibit the release of arachidonic acid, thereby inhibiting the biosynthesis of potent mediators of inflammation, such as prostaglandins and leukotrienes.
Loteprednol etabonate is an etabonate ester, an 11beta-hydroxy steroid, a steroid ester, an organochlorine compound, a steroid acid ester and a 3-oxo-Delta(1),Delta(4)-steroid. It has a role as an anti-inflammatory drug. It derives from a loteprednol.
Loteprednol Etabonate (LE) is a topical corticoid anti-inflammatory. It is used in ophthalmic solution for the treatment of steroid responsive inflammatory conditions of the eye such as allergic conjunctivitis, uveitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, and selected infective conjunctivitides. As a nasal spray, it can be used for the treatment and management of seasonal allergic rhinitis. Most prescription LE products, however, tend to be indicated for the treatment of post-operative inflammation and pain following ocular surgery. A number of such new formulations that have been approved include Kala Pharmaceutical’s Inveltys – the first twice-daily (BID) ocular corticosteroid approved for this indication, designed specifically to enhance patient compliance and simplified dosing compared to all other similar ocular steroids that are dosed four times daily. Moreover, LE was purposefully engineered to be a ‘soft drug’, one that is designed to be active locally at the site of administration and then rapidly metabolized to inactive components after eliciting its actions at the desired location, thereby subsequently minimizing the chance for adverse effects.
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2012-09-28 | New dosage form | Lotemax | Postoperative inflammation and pain following ocular surgery | Gel | 0.5% | Bausch & Lomb | |
| 2011-04-15 | New dosage form | Lotemax | Postoperative inflammation and pain following ocular surgery | Ointment | 0.5% | Bausch & Lomb | |
| 1998-03-09 | First approval | Lotemax | Allergic conjunctivitis,Acne rosacea,Superficial punctate keratitis,Herpes zoster keratitis,Iritis,Cyclitis | Suspension/ Drops | 0.5% | Bausch & Lomb |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2014-11-26 | Marketing approval | 露达舒/Lotemax | Allergic conjunctivitis,Acne rosacea,Superficial punctate keratitis,Herpes zoster keratitis,Iritis,Cyclitis,Postoperative inflammation and pain following ocular surgery | Suspension | 滴眼剂,0.5%(2.5ml:12.5mg,5ml:25mg) | Bausch & Lomb | |
| 2011-11-05 | Marketing approval | 露达舒/Lotemax | Allergic conjunctivitis,Acne rosacea,Superficial punctate keratitis,Herpes zoster keratitis,Iritis,Cyclitis,Postoperative inflammation and pain following ocular surgery | Suspension | 滴眼剂,0.5%(2.5ml:12.5mg,5ml:25mg); 滴眼剂,0.5%(10ml:50mg,15ml:75mg) | Bausch & Lomb |
Reference:1. US4710495A / US4996335A.Route 2
Reference:1. CN103183714A.
SYN
doi:10.1016/0960-0760(91)90120-T doi: 10.1016/j.steroids.2011.01.006

| Clinical data | |
|---|---|
| Trade names | Lotemax |
| Other names | 11β,17α,Dihydroxy-21-oxa-21-chloromethylpregna-1,4-diene-3,20-dione 17α-ethylcarbonate |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Eye drops |
| Drug class | Corticosteroid; glucocorticoid |
| ATC code | S01BA14 (WHO) |
| Legal status | |
| Legal status | US: ℞-only |
| Pharmacokinetic data | |
| Bioavailability | None |
| Protein binding | 95% |
| Metabolism | Ester hydrolysis |
| Metabolites | Δ1-cortienic acid and its etabonate |
| Onset of action | ≤2 hrs (allergic conjunctivitis) |
| Elimination half-life | 2.8 hrs |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 82034-46-6 |
| PubChem CID | 444025 |
| IUPHAR/BPS | 7085 |
| DrugBank | DB14596 |
| ChemSpider | 392049 |
| UNII | YEH1EZ96K6 |
| KEGG | D01689 |
| ChEBI | CHEBI:31784 |
| ChEMBL | ChEMBL1200865 |
| CompTox Dashboard (EPA) | DTXSID2046468 |
| ECHA InfoCard | 100.167.120 |
| Chemical and physical data | |
| Formula | C24H31ClO7 |
| Molar mass | 466.96 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| Melting point | 220.5 to 223.5 °C (428.9 to 434.3 °F) |
| Solubility in water | 0.0005 mg/mL (20 °C) |
| showSMILES | |
| showInChI | |
| (what is this?) (verify) |

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com
////////////////////////////////////////
Medical uses
Applications for this drug include the reduction of inflammation after eye surgery,[1] seasonal allergic conjunctivitis, uveitis,[3] as well as chronic forms of keratitis (e.g. adenoviral and Thygeson’s keratitis), vernal keratoconjunctivitis, pingueculitis, and episcleritis.[citation needed]
Contraindications
As corticosteroids are immunosuppressive, loteprednol is contraindicated in patients with viral, fungal or mycobacterial infections of the eye.[1][3][4]
Adverse effects
The most common adverse effects in patients being treated with the gel formulation are anterior chamber inflammation (in 5% of people), eye pain (2%), and foreign body sensation (2%).[5]
Interactions
Because long term use (more than 10 days) can cause increased intraocular pressure, loteprednol may interfere with the treatment of glaucoma. Following ocular administration, the drug is very slowly absorbed into the blood, therefore the blood level is limited to an extremely small concentration, and interactions with drugs taken by mouth or through any route other than topical ophthalmic are very unlikely.[1]
Pharmacology
Mechanism of action
Main article: Glucocorticoid § Mechanism of action
Pharmacokinetics
Neither loteprednol etabonate nor its inactive metabolites Δ1–cortienic acid and Δ1-cortienic acid etabonate are detectable in the bloodstream, even after oral administration. A study with patients receiving loteprednol eye drops over 42 days showed no adrenal suppression, which would be a sign of the drug reaching the bloodstream to a clinically relevant extent.[1]
Steroid receptor affinity was 4.3 times that of dexamethasone in animal studies.[1]
Retrometabolic drug design
Loteprednol etabonate was developed using retrometabolic drug design. It is a so-called soft drug, meaning its structure was designed so that it is predictably metabolised to inactive substances. These metabolites, Δ1-cortienic acid and its etabonate, are derivatives of cortienic acid, itself an inactive metabolite of hydrocortisone.[1][4][6]
- Cortisol, a naturally occurring corticosteroid, known as hydrocortisone when used as a drug
- Δ1-Cortienic acid, inactive metabolite of loteprednol
- Cortienic acid, inactive metabolite of hydrocortisone
Chemistry
Loteprednol etabonate is an ester of loteprednol with etabonate (ethyl carbonate). The pure chemical compound has a melting point between 220.5 °C (428.9 °F) and 223.5 °C (434.3 °F). Its solubility in water is 1:2,000,000,[4] therefore it is formulated for ophthalmic use as either an ointment, a gel, or a suspension.[7]
Loteprednol is a corticosteroid. The ketone side chain of classical corticosteroids such as hydrocortisone is replaced by a cleavable ester, which accounts for the rapid inactivation.[8] (This is not the same as the etabonate ester.)

Loteprednol etabonate
Chemical synthesis
References
- ^ Jump up to:a b c d e f g Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 488. ISBN 9783527607495.
- ^ Jump up to:a b Loteprednol Professional Drug Facts.
- ^ Jump up to:a b c Dinnendahl V, Fricke U (2008). Arzneistoff-Profile (in German). 6 (22 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ “Highlights of Prescribing Information: Lotemax” (PDF). 2012.
- ^ Bodor N, Buchwald P (2002). “Design and development of a soft corticosteroid, loteprednol etabonate”. In Schleimer RP, O’Byrne PM, Szefler SJ, Brattsand R (eds.). Inhaled Steroids in Asthma. Optimizing Effects in the Airways. Lung Biology in Health and Disease. 163. Marcel Dekker, New York. pp. 541–564.
- ^ “Loteprednol (Professional Patient Advice)”. Retrieved October 4, 2018.
- ^ Pavesio CE, Decory HH (April 2008). “Treatment of ocular inflammatory conditions with loteprednol etabonate”. The British Journal of Ophthalmology. 92 (4): 455–9. doi:10.1136/bjo.2007.132621. PMID 18245274. S2CID 25873047.
- ^ Druzgala P, Hochhaus G, Bodor N (February 1991). “Soft drugs–10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate”. The Journal of Steroid Biochemistry and Molecular Biology. 38 (2): 149–54. doi:10.1016/0960-0760(91)90120-T. PMID 2004037. S2CID 27107845.
Further reading
- Stewart R, Horwitz B, Howes J, Novack GD, Hart K (November 1998). “Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1”. Journal of Cataract and Refractive Surgery. 24 (11): 1480–9. doi:10.1016/s0886-3350(98)80170-3. PMID 9818338. S2CID 24423725.
////////////loteprednol etabonate
CCOC(=O)OC1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)C(=O)OCCl
