Clik here to view.
Clik here to view.

- Molecular FormulaC12H17NO2
- Average mass207.269 Da
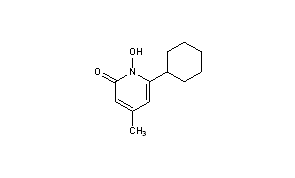
CICLOPIROX(6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone)
2(1H)-Pyridinone, 6-cyclohexyl-1-hydroxy-4-methyl-
249-577-2[EINECS]
29342-05-0[RN]
KS-5085, циклопирокс , سيكلوبيروكس , 环吡酮 ,
Ciclopirox
CAS Registry Number: 29342-05-0
CAS Name: 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone
Molecular Formula: C12H17NO2
Molecular Weight: 207.27
Percent Composition: C 69.54%, H 8.27%, N 6.76%, O 15.44%
Literature References: Broad spectrum antimycotic agent with some antibacterial activity. Prepn: G. Lohaus, W. Dittmar, ZA6906039; eidem,US3883545 (1970, 1975 both to Hoechst). In vitro study: eidem,Arzneim.-Forsch.23, 670 (1973). Series of articles on pharmacokinetics, pharmacology, teratology, toxicity studies: Oyo Yakuri9, 57-115 (1975), C.A.83, 53159d, 53538b, 53539c, 71844c, 90833q (1975). Series of articles on chemistry, mechanism of action, toxicology, clinical trials: Arzneim.-Forsch.31, 1309-1386 (1981). Toxicity data: H. G. Alpermann, E. Schutz, ibid. 1328. Review: S. G. Jue et al.,Drugs29, 330-341 (1985). Review of clinical experience in seborrheic dermatitis: A. Starova, R. Aly, Expert Opin. Drug Saf.4, 235-239 (2005).
Properties: Solid, mp 144°.
Melting point: mp 144°
Clik here to view.
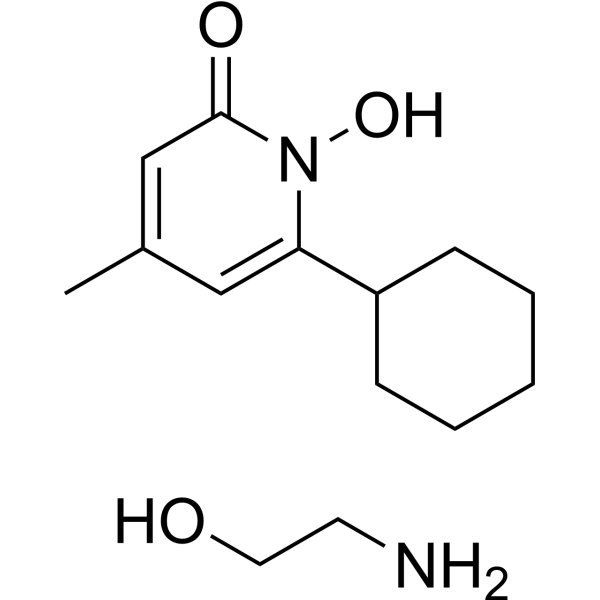
Derivative Type: Ethanolamine salt (1:1)
CAS Registry Number: 41621-49-2
Additional Names: Ciclopirox olamine
Manufacturers’ Codes: HOE-296
Trademarks: Batrafen (HMR); Brumixol (Bruschettini); Ciclochem (Novag); Dafnegin (Poli); Loprox (HMR); Micoxolamina (Domp?; Mycoster (Fabre)
Molecular Formula: C14H24N2O3
Molecular Weight: 268.35
Percent Composition: C 62.66%, H 9.01%, N 10.44%, O 17.89%
Properties: LD50 in mice, rats (mg/kg): 2898, 3290 orally (Alpermann, Schutz).
Toxicity data: LD50 in mice, rats (mg/kg): 2898, 3290 orally (Alpermann, Schutz)
- EINECS:255-464-9
- LD50:71 mg/kg (M, i.v.); 1740 mg/kg (M, p.o.);
72 mg/kg (R, i.v.); 2350 mg/kg (R, p.o.)
Therap-Cat: Antifungal.
Keywords: Antifungal (Synthetic).
Clik here to view.

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE
Clik here to view.

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
Ciclopirox (sometimes known by the abbreviation CPX[2]) is a synthetic antifungal agent for topical dermatologic treatment of superficial mycoses. It is most useful against Tinea versicolor. It is sold under many brand names worldwide.[1]
Medical uses
Ciclopirox is indicated for the treatment of tinea pedis and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes and Epidermophyton floccosum, as well as seborrheic dermatitis. It is not to be used in the eyes or vagina, and nursing women should consult their doctors before use, since it is not known whether ciclopirox passes into human milk. A burning sensation may be felt when first applying ciclopirox on the skin.[3]
Nail infections
In addition to other formulations, ciclopirox is used in lacquers for topical treatment of onychomycosis (fungal infections of the nails). A meta-analysis of the six trials of nail infections available in 2009 concluded that they provided evidence that topical ciclopirox had poor cure rates and that amorolfine might be substantially more effective, but more research was required.
“Combining data from 2 trials of ciclopiroxolamine versus placebo found treatments failure rates of 61% and 64% for ciclopiroxolamine. These outcomes followed long treatment times (48 weeks) and this makes ciclopiroxolamine a poor choice for nail infections. Better results were observed with the use of amorolfine lacquer; 6% treatment failure rates were found after 1 month of treatment but these data were collected on a very small sample of people and these high rates of success might be unreliable.”[4]
Pharmacology
Pharmacodynamics
In contrast to the azoles and other antimycotic drugs, the mechanism of action of ciclopirox is poorly understood.[5] However, loss of function of certain catalase and peroxidase enzymes has been implicated as the mechanism of action, as well as various other components of cellular metabolism. In a study conducted to further elucidate ciclopirox’s mechanism, several Saccharomyces cerevisiae mutants were screened and tested. Results from interpretation of the effects of both the drug treatment and mutation suggested that ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport.[6]
It is currently being investigated as an alternative treatment to ketoconazole for seborrhoeic dermatitis as it suppresses growth of the yeast Malassezia furfur. Initial results show similar efficacy to ketoconazole with a relative increase in subjective symptom relief due to its inherent anti-inflammatory properties.[7]
Chemistry
Ciclopirox is a considered a hydroxypyrimidine (sic) antifungal agent.[citation needed] Structurally, ciclopirox is the N-oxide of a 2-hydroxypyridine derivative and therefore ought to be termed a hydroxypyridine antifungal agent. Additionally, the structure as drawn above is the lactam tautomer and indicates the molecule being an N-Hydroxy-2-pyridone. Hence the classification of ciclopirox as a 2-pyridone antifungal agent.
Ciclopirox is used clinically as ciclopirox olamine, the olamine salt of ciclopirox.
Literatures:
Lohaus; Dittmar Arzneimittel-Forschung/Drug Research, 1981 , vol. 31, # 8 a p. 1311 – 1316
Literatures:
Hoechst Aktiengesellschaft Patent: US3972888 A1, 1976 ;
Clik here to view.

Clik here to view.

Clik here to view.

SYNTHESIS
SYN
Clik here to view.

SYN
W. Dittmar, E. Druckrey andBroad spectrum antimycotic agent with some antibacterial activity. Prepn: G. Lohaus, W. Dittmar, ZA 6906039; eidem, US 3883545 (1970, 1975 both to Hoechst). In vitro study: eidem, Arzneim.-Forsch. 23, 670 (1973).
H. Urbach, J. Med. Chem., 17, 753 (1974); W. Dittmar and G. Lohaus,
German Patent 2,214,608 (1973); Chem. Abstr., 79: 146419w (1973).
Clik here to view.

SYN
ethanolamine (CAS NO.: ), with other names as 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone 2-aminoethanol, could be produced through the following synthetic routes.
Clik here to view.
.jpg)
Compound can be prepared in two different ways: 1) The reaction of methyl 5-oxo-5-cyclohexyl-3-methylpentenoate (I) with NH2OH gives the corresponding oxime (II), which is then cyclized to 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone (III). Finally, this compound is salified with ethanolamine (IV). 2) Compound (III) can also be obtained by reaction of 6-cyclohexyl-4-methyl-2-pyron (V) with hydroxylamine hydrochloride in hot 2-aminopyridine.
SYN
Clik here to view.
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 14818-35-0 | C12H16O2 | 6-cyclohexyl-4-methyl-2-pyrone | 2H-Pyran-2-one, 6-cyclohexyl-4-methyl- |
| 141-43-5 | C2H7NO | ethanolamine | Ethanol, 2-amino- |
| 7803-49-8 | H3NO | hydroxylamine | Hydroxylamine |
PATENT
https://patents.google.com/patent/US9545413B2/en
The molecule 6-cyclohexyl-1-hydroxy-4-methylpyridin-2(1H)-one, also known as Ciclopirox, is a commercially available antifungal agent as an olamine salt. Ciclopirox olamine has been used to treat superficial mycoses and Tinea versicolor following topical application to the skin. Following enteral administration, ciclopirox undergoes significant first-pass effect resulting in low oral bioavailability. The oral route of administration is also associated with gastrointestinal toxicities observed in animals and humans limiting its benefit in animal and human health applications. Ciclopirox olamine has poor solubility, limiting opportunities to deliver the antifungal agent via parenteral administration of suitably potent solutions and suspensions. As such, it would be beneficial to configure ciclopirox for improved water solubility in order to deliver the drug by parenteral routes of administration.
References
- ^ Jump up to:a b Drugs.com International brand names for ciclopirox Page accessed January 201, 2016
- ^ Ciclopirox
- ^ “Ciclopirox Olamine Antifungal Shampoo”. Okdermo. Retrieved 2019-08-06.
- ^ Crawford F (2007). “Topical treatments for fungal infections of the skin and nails of the foot”. The Cochrane Database of Systematic Reviews. 2007 (3): CD001434. doi:10.1002/14651858.CD001434.pub2. PMC 7073424. PMID 17636672.
- ^ Niewerth M, Kunze D, Seibold M, Schaller M, Korting HC, Hube B (June 2003). “Ciclopirox Olamine Treatment Affects the Expression Pattern of Candida albicans Genes Encoding Virulence Factors, Iron Metabolism Proteins, and Drug Resistance Factors”. Antimicrobial Agents and Chemotherapy. 47 (6): 1805–1817. doi:10.1128/AAC.47.6.1805-1817.2003. PMC 155814. PMID 12760852.
- ^ Leem SH, Park JE, Kim IS, Chae JY, Sugino A, Sunwoo Y (2003). “The possible mechanism of action of ciclopirox olamine in the yeast Saccharomyces cerevisiae”. Mol. Cells. 15 (1): 55–61. PMID 12661761.
- ^ Ratnavel RC, Squire RA, Boorman GC (2007). “Clinical efficacies of shampoos containing ciclopirox olamine (1.5%) and ketoconazole (2.0%) in the treatment of seborrhoeic dermatitis”. J Dermatolog Treat. 18 (2): 88–96. doi:10.1080/16537150601092944. PMID 17520465. S2CID 34852507.
| Clinical data | |
|---|---|
| Trade names | Many brand names worldwide[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a604021 |
| Pregnancy category | B |
| Routes of administration | Topical (applied as a nail lacquer, skin cream or shampoo) |
| ATC code | D01AE14 (WHO) G01AX12 (WHO) |
| Legal status | |
| Legal status | US: ℞-onlyRx-only (CA) |
| Pharmacokinetic data | |
| Bioavailability | <5% with prolonged use |
| Protein binding | 94 to 97% |
| Elimination half-life | 1.7 hours |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 29342-05-0 |
| PubChem CID | 2749 |
| DrugBank | DB01188 |
| ChemSpider | 2647 |
| UNII | 19W019ZDRJ |
| KEGG | D03488 |
| ChEBI | CHEBI:453011 |
| ChEMBL | ChEMBL1413 |
| CompTox Dashboard (EPA) | DTXSID9048564 |
| ECHA InfoCard | 100.045.056 |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.269 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (verify) |
////////CICLOPIROX OLAMINE
Clik here to view.
