
VX 148
297730-05-3
Name: VX-148
CAS#: 297730-05-3
Chemical Formula: C23H25N5O4
Exact Mass: 435.19065
Molecular Weight: 435.48
Elemental Analysis: C, 63.44; H, 5.79; N, 16.08; O, 14.70
| Molecular Weight | 435.48 |
| Formula | C23H25N5O4 |
| CAS No. | 297730-05-3 (VX 148); |
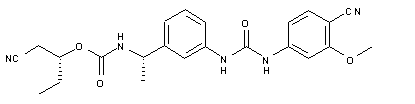
| Chemical Name | Carbamic acid, N-[(1S)-1-[3-[[[(4-cyano-3-methoxyphenyl)amino]carbonyl]amino]phenyl]ethyl]-, (1R)-1-(cyanomethyl)propyl ester |
- OriginatorVertex Pharmaceuticals
- ClassAntipsoriatics
- Mechanism of ActionInosine monophosphate dehydrogenase inhibitors
- DiscontinuedPsoriasis; Transplant rejection; Viral infections
- 13 Nov 2003Interim data from a media release have been added to the adverse events and Skin Disorders therapeutic trials sections
- 23 May 2003Vertex Pharmaceuticals has completed enrolment in a phase IIa trial for Psoriasis in Iceland
- 24 Dec 2002Phase-II clinical trials in Psoriasis in Iceland (unspecified route)
VX-148 is a second-generation, orally administered inhibitor of inosine monophosphate dehydrogenase (IMPDH). The IMPDH enzyme plays a key role in regulating immune response and proliferation of specific cell types, including lymphocytes. VX-148 is a developed for the treatment of autoimmune diseases.
Investigated for use/treatment in autoimmune diseases, psoriasis and psoriatic disorders, and viral infection.
VX-148 is a novel, uncompetitive IMPDH inhibitor with a K(i) value of 6 nM against IMPDH type II enzyme. VX-148 is slightly more potent than mycophenolic acid and VX-497 in inhibiting the proliferation of mitogen-stimulated primary human lymphocytes (IC(50) value of ~80 nM). The inhibitory activity of VX-148 is alleviated in the presence of exogenous guanosine. VX-148 does not inhibit proliferation of nonlymphoid cell types such as fibroblasts, indicating selectivity for inhibition of IMPDH activity. VX-148 is orally bioavailable in rats and mice; oral administration of VX-148 inhibits primary antibody response in mice in a dose-dependent manner with an ED(50) value of 38 mg/kg b.i.d. VX-148 significantly prolongs skin graft survival at 100 mg/kg b.i.d. in mice.
SYN
WO 0056331
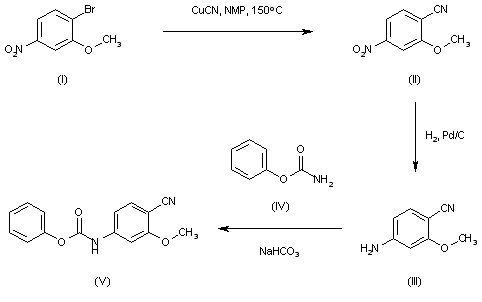
The intermediate carbamate (V) has been obtained as follows. The reaction of 4-bromo-3-methoxynitrobenzene (I) with CuCN in NMP at 150 C gives 2-methoxy-4-nitrobenzonitrile (II), which is reduced with H2 over Pd/C in ethyl acetate to yield 4-amino-2-methoxybenzonitrile (III). Finally, this compound is condensed with phenyl carbamate (IV) by means of NaHCO3 in ethyl acetate to afford the desired carbamate intermediate (V).
SYN
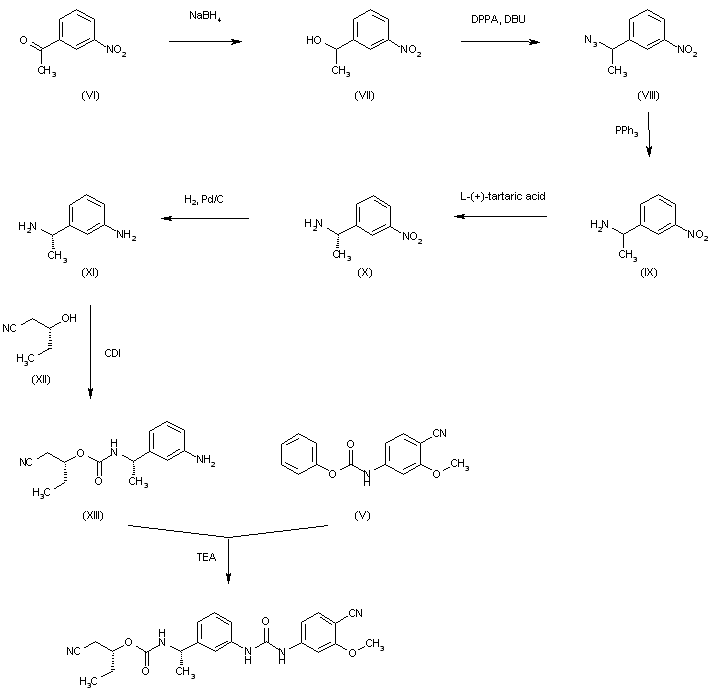
The reduction of 3-nitroacetophenone (VI) by means of NaBH4 in ethanol gives 1-(3-nitrophenyl)ethanol (VII), which is treated with DPPA and DBU in hot toluene to yield the azido derivative (VIII). The reduction of (VIII) with PPh3 in THF/water affords 1-(3-nitrophenyl)ethylamine (IX) as a racemic mixture that is submitted to optical resolution with L-(+)-tartaric acid to provide the desired (S)-isomer (X). The reduction of the nitro group of (X) by means of H2 over Pd/C in methanol gives 1(S)-(3-aminophenyl)ethylamine (XI), which is condensed with 2(R)-hydroxypentanenitrile (XII) and CDI to yield the carbamate (XIII). Finally, this compound is condensed with intermediate carbamate (V) by means of TEA in hot ethyl acetate to afford the target urea.
- Jain J, Almquist SJ, Heiser AD, Shlyakhter D, Leon E, Memmott C, Moody CS, Nimmesgern E, Decker C: Characterization of pharmacological efficacy of VX-148, a new, potent immunosuppressive inosine 5′-monophosphate dehydrogenase inhibitor. J Pharmacol Exp Ther. 2002 Sep;302(3):1272-7. [Article]
////////////VX 148, phase 2
O=C(O[C@H](CC)CC#N)N[C@H](C1=CC=CC(NC(NC2=CC=C(C#N)C(OC)=C2)=O)=C1)C
