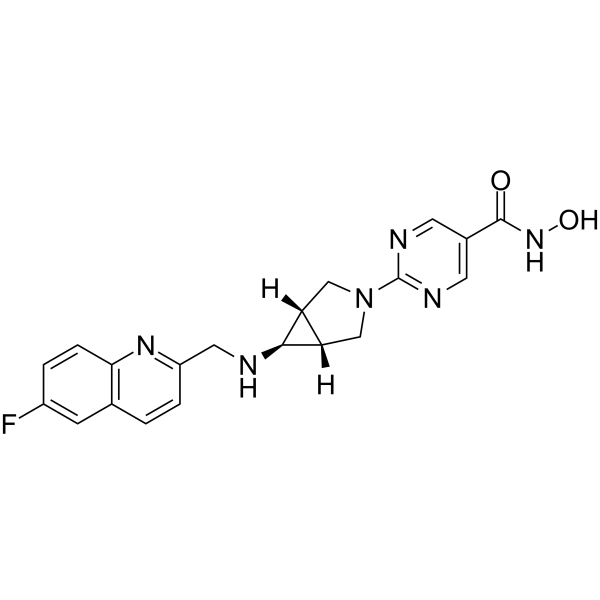

Nanatinostat
Tractinostat
CHR-3996, CHR 3996, VRx 3996,
C20H19FN6O2, 394.41
CAS 1256448-47-1
2-[(1α,5α,6α)-6-[[(6-Fluoro-2-q
2-[(1R,5S,6R)-6-{[(6-fluoroquinolin-2-yl)methyl]amino}-3-azabicyclo[3.1.0]hexan-3-yl]-N-hydroxypyrimidine-5-carboxamide2-[(1R,5S,6s)-6-{[(6-Fluoro-2-quinolinyl)methyl]amino}-3-azabicyclo[3.1.0]hex-3-yl]-N-hydroxy-5-pyrimidinecarboxamide5-Pyrimidinecarboxamide, 2-[(1R,5S)-6-[[(6-fluoro-2-quinolinyl)methyl]amino]-3-azabicyclo[3.1.0]hex-3-yl]-N-hydroxy-Chroma Therapeutics Ltd. (Originator)
- OriginatorChroma Therapeutics
- DeveloperChroma Therapeutics; Viracta Therapeutics
- ClassAmides; Antineoplastics; Pyrimidines; Quinolines; Small molecules
- Mechanism of ActionHistone deacetylase inhibitors
- Orphan Drug StatusYes – Post-transplant lymphoproliferative disorder; Plasmablastic lymphoma; T-cell lymphoma
- Phase IILymphoma
- Phase I/IIMultiple myeloma
- Phase ISolid tumours
- No development reportedGastric cancer; Nasopharyngeal cancer; Post-transplant lymphoproliferative disorder
- 01 Jun 2021Phase-II clinical trials in Lymphoma (Combination therapy, Second-line therapy or greater) in North America, Europe, Asia (PO)
- 18 May 2021Ninatinostat is still in phase I trials for Solid tumour in United Kingdom and Netherlands (Viracta Therapeutics pipeline, May 2021)
- 18 May 2021Virata Therapeutics has patent protection for dose regimen in NAVAL-1 trial in USA
Nanatinostat is under investigation in clinical trial NCT00697879 (Safety Study of the Histone Deacetylase Inhibitor, CHR-3996, in Patients With Advanced Solid Tumours).
Nanatinostat is an orally bioavailable, second-generation hydroxamic acid-based inhibitor of histone deacetylase (HDAC), with potential antineoplastic activity. Nanatinostat targets and inhibits HDAC, resulting in an accumulation of highly acetylated histones, the induction of chromatin remodeling, and the selective transcription of tumor suppressor genes; these events result in the inhibition of tumor cell division and the induction of tumor cell apoptosis. This agent may upregulate HSP70 and downregulate anti-apoptotic Bcl-2 proteins more substantially than some first-generation HDAC inhibitors. HDACs, upregulated in many tumor cell types, are a family of metalloenzymes responsible for the deacetylation of chromatin histone proteins.

Patent
WO2006123121
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2006123121
Example 44: N-Hvdroxy 2-(6-fr(6-fluoroαuinolin-2-yl)methvnamino)-3-azabicvclorS.I.OIhex-S-vDpyrimidine-δ-carboxamide
LCMS purity >98%, m/z 395 [M+H]+, 1H NMR (300 MHz, c/6-DMSO) δ: 2.30 (2H, s), 2.75 (1 H, s), 3.60 (2H, dm, J = 11.7 Hz), 3.88 (2H, d, J = 11.7 Hz), 4.69 (2H, br s), 7.66 (1 H, d, J = 8.4 Hz), 7.75 (1 H, td, J = 8.7, 3.0 Hz), 7.88 (1 H, dd, J = 9.3, 2.7 Hz), 8.48 (1 H, d, J = 8.4 Hz), 8.67 (2H, s), 9.01 (1 H, br s), 9.61 (1 H, br s), 11.09 (1 H, br s).
PATENT
WO-2021113694
Crystalline hydrate form A of N-hydroxy 2-{6-[(6-fluoro-quinolin-2-ylmethyl)-amino]-3-aza-bicyclo[3.1.0]hex-3-yl}pyrimidine-5-carboxamide ( nanatinostat ) .
Compound 1 is also known as nanatinostat, VRx-3996, or CHR-3996. It has been previously described in patents and patent applications, e.g. US patent 7,932,246 and US patent application 15/959,482, each of which is incorporated by reference in their entirety.
Compound 1
PATENT
WO2021071809 , claiming dosages for HDAC treatment with reduced side effects.
/////////Nanatinostat, CHR-3996, CHR 3996, VRx 3996, CHROMA, ORPHAN DRUG, Tractinostat, PHASE 2
| FC1=CC=C2N=C(CN[C@H]3[C@]4([H])CN(C5=NC=CC(C(NO)=O)=N5)C[C@]34[H])C=CC2=C1 |
