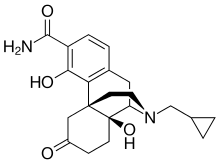

Samidorphan
サミドルファン;
| Formula | C21H26N2O4 |
|---|---|
| CAS | 852626-89-2 |
| Mol weight | 370.4421 |
FDA APPROVED 5/28/2021 Lybalvi
- ALKS 33
- ALKS-33
- RDC-0313
- RDC-0313-00
Product Ingredients
UNII0AJQ5N56E0
CAS Number1204592-75-5
WeightAverage: 504.536
Monoisotopic: 504.210780618
Chemical FormulaC25H32N2O9
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Samidorphan L-malate | 0AJQ5N56E0 | 1204592-75-5 | RARHXUAUPNYAJF-QSYGGRRVSA-N |
IUPAC Name(1R,9R,10S)-17-(cyclopropylmethyl)-3,10-dihydroxy-13-oxo-17-azatetracyclo[7.5.3.0^{1,10}.0^{2,7}]heptadeca-2,4,6-triene-4-carboxamide; (2S)-2-hydroxybutanedioic acid
MOA:mu-Opioid antagonist; delta-Opioid partial agonist; kappa-Opioid partial agonistsIndication:Alcohol dependence
New Drug Application (NDA): 213378
Company: ALKERMES INChttps://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213378s000lbl.pdfhttps://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/213378Orig1s000,%20Orig2s000ltr.pdf
To treat schizophrenia in adults and certain aspects of bipolar I disorder in adults
LYBALVI is a combination of olanzapine, an atypical antipsychotic, and samidorphan (as samidorphan L-malate), an opioid antagonist.
Olanzapine is 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine. The molecular formula of olanzapine is: C17H20N4S and the molecular weight is 312.44 g/mol. It is a yellow crystalline powder and has pKa values of 7.80 and 5.44. The chemical structure is:
 |
Samidorphan L-malate is morphinan-3-carboxamide, 17-(cyclopropylmethyl)-4, 14-dihydroxy-6-oxo-, (2S)-2-hydroxybutanedioate. The molecular formula of samidorphan L-malate is C21H26N2O4 • C4H6O5 and the molecular weight is 504.54 g/mol. It is a white to off-white crystalline powder and has pKa values of 8.3 (amine) and 10.1 (phenol). The chemical structure is:
 |
LYBALVI is intended for oral administration and is available as film-coated, bilayer tablets in the following strengths: 5 mg/10 mg, 10 mg/10 mg, 15 mg/10 mg, and 20 mg/10 mg of olanzapine and samidorphan (equivalent to 13.6 mg of samidorphan L-malate).
Inactive ingredients include colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The film coating ingredients include hypromellose, titanium dioxide, triacetin, and color additives [iron oxide yellow (5 mg/10 mg); iron oxide yellow and iron oxide red (10 mg/10 mg); FD&C Blue No. 2/ indigo carmine aluminum lake (15 mg/10 mg); iron oxide red (20 mg/10 mg)].
- to treat schizophrenia
- alone for short-term (acute) or maintenance treatment of manic or mixed episodes that happen with bipolar I disorder
- in combination with valproate or lithium to treat manic or mixed episodes that happen with bipolar I disorder
Olanzapine is an effective atypical antipsychotic that, like other antipsychotics, is associated with weight gain, metabolic dysfunction, and increased risk of type II diabetes.5,6 Samidorphan is a novel opioid antagonist structurally related to naltrexone, with a higher affinity for opioid receptors, more potent μ-opioid receptor antagonism, higher oral bioavailability, and a longer half-life, making it an attractive candidate for oral dosing.1,5,11 Although antipsychotic-induced weight gain is incompletely understood, it is thought that the opioid system plays a key role in feeding and metabolism, such that opioid antagonism may be expected to ameliorate these negative effects. Samidorphan has been shown in animal models and clinical trials to ameliorate olanzapine-induced weight gain and metabolic dysfunction.5,6
Samidorphan was first approved as a variety of fixed-dose combination tablets with olanzapine by the FDA on May 28, 2021, and is currently marketed under the trademark LYBALVI by Alkermes Inc.11
by Alkermes Inc.11
Samidorphan (INN, USAN) (developmental code names ALKS-33, RDC-0313), also known as 3-carboxamido-4-hydroxynaltrexone,[2] is an opioid antagonist that preferentially acts as an antagonist of the μ-opioid receptor (MOR). It is under development by Alkermes for the treatment of major depressive disorder and possibly other psychiatric conditions.[3]
Development
Samidorphan has been investigated for the treatment of alcoholism and cocaine addiction by its developer, Alkermes,[4][5] showing similar efficacy to naltrexone but possibly with reduced side effects.
However, it has attracted much more attention as part of the combination product ALKS-5461 (buprenorphine/samidorphan), where samidorphan is combined with the mixed MOR weak partial agonist and κ-opioid receptor (KOR) antagonist buprenorphine, as an antidepressant. Buprenorphine has shown antidepressant effects in some human studies, thought to be because of its antagonist effects at the KOR, but has not been further developed for this application because of its MOR agonist effects and consequent abuse potential. By combining buprenorphine with samidorphan to block the MOR agonist effects, the combination acts more like a selective KOR antagonist, and produces only antidepressant effects, without typical MOR effects such as euphoria or substance dependence being evident.[6][7]
Samidorphan is also being studied in combination with olanzapine, as ALKS-3831 (olanzapine/samidorphan), for use in schizophrenia.[8] A Phase 3 study found that the addition of samidorphan to olanzapine significantly reduced weight gain compared to olanzapine alone.[9] The combination is now under review for approval by the US Food and Drug Administration.[10]
Pharmacology
Pharmacodynamics
The known activity profile of samidorphan at the opioid receptors is as follows:[11][12]
- μ-Opioid receptor (Ki = 0.052 nM; EC50 = N/A; Emax = 3.8%; IC50 = 0.88 nM; Imax = 92%)
- κ-Opioid receptor (Ki = 0.23 nM; EC50 = 3.3 nM; Emax = 36%; IC50 = 38 nM; Imax = 57%)
- δ-Opioid receptor (Ki = 2.6 nM; EC50 = 1.5 nM; Emax = 35%; IC50 = 6.9 nM; Imax = 56%)
As such, samidorphan is primarily an antagonist, or extremely weak partial agonist of the MOR.[11][12] In accordance with its in vitro profile, samidorphan has been observed to produce some side effects that are potentially consistent with activation of the KOR such as somnolence, sedation, dizziness, and hallucinations in some patients in clinical trials at the doses tested.[13]
SYNPATENT
WO2006052710A1.
https://patents.google.com/patent/WO2006052710A1/enExample 1 -Synthesis of 3-Carboxyamido-4-hvdroxy-naltrexone derivative 3

(A) Synthesis of 3-Carboxyamido-naltrexone 2[029] The triflate 11 of naltrexone was prepared according to the method of Wentland et al. (Bioorg. Med. Chem. Lett. 9, 183-187 (2000)), and the carboxamide 2 was prepared by the method described by Wentland et al. [(Bioorg. Med. Chem. Lett. ϋ, 623-626 (2001); and Bioorg. Med. Chem. Lett. 11, 1717-1721 (2001)] involving Pd-catalyzed carbonylation of the triflate 11 in the presence of ammonia and the Pd(O) ligand, DPPF ([l,l’-bis(diphenylρhosphino)ferrocene]) and DMSO.(B) Synthesis of 3-Carboxyamido-4-hydroxy-naltrexone derivative 3[030] Zinc dust (26 mg, 0.40 mmol) was added in portions to a solution of 2 (50 mg, 0.14 mmol) in HCl (37%, 0.2 mL) and AcOH (2 mL) at reflux. After heating at reflux for a further 15 min, the reaction was cooled by the addition of ice/water (10 mL) and basified (pH=9) with NH3/H2O, and the solution was extracted with EtOAc (3×10 mL). The organic extracts were washed with brine, dried, and concentrated. The residue was purified by column chromatography (SiO2, CH2Cl2, CH3OH : NH3/H2O = 15:1:0.01) to give compound 3 as a foam (25 mg, 50%). 1H NMR (CDC13) δl3.28(s, IH, 4-OH), 7.15(d, IH, J=8.1, H-2), 6.47(d, IH, J=8.4, H- 1), 6.10(br, IH, N-H), 4.35(br, IH, N-H), 4.04(dd,lH, J=I.8, 13.5, H-5), 3.11( d, IH, J=6), 2.99( d, IH, J=5.7), 2.94( s, IH), 2.86( d, IH, J= 6), 2.84-2.75(m, 2H), 2.65-2.61(m, 2H), 2.17-2.05(m, IH), 1.89-1.84(m, 2H), 0.85(m, IH), 0.56-0.50(m, 2H), 0.13-0.09(m, 2H). [α]D25= -98.4° (c=0.6, CH2Cl2). MS m/z (ESI) 371(MH+).
Paper
Bioorg. Med. Chem. Lett. 2000, 10, 183-187.
https://www.sciencedirect.com/science/article/abs/pii/S0960894X99006708
Abstract
Opioid binding affinities were assessed for a series of cyclazocine analogues where the prototypic 8-OH substituent of cyclazocine was replaced by amino and substituted-amino groups. For μ and κ opioid receptors, secondary amine derivatives having the (2R,6R,11R)-configuration had the highest affinity. Most targets were efficiently synthesized from the triflate of cyclazocine or its enantiomers using Pd-catalyzed amination procedures.
PAPER
Bioorg. Med. Chem. Lett. 2001, 11, 1717-1721.
https://www.sciencedirect.com/science/article/abs/pii/S0960894X01002785
Abstract
In response to the unexpectedly high affinity for opioid receptors observed in a novel series of cyclazocine analogues where the prototypic 8-OH was replaced by a carboxamido group, we have prepared the corresponding 3-CONH2 analogues of morphine and naltrexone. High affinity (Ki=34 and 1.7 nM) for μ opioid receptors was seen, however, the new targets were 39- and 11-fold less potent than morphine and naltrexone, respectively.
Abstract
High-affinity binding to μ opioid receptors has been identified in a series of novel 3-carboxamido analogues of morphine and naltrexone.

References
- ^ Turncliff R, DiPetrillo L, Silverman B, Ehrich E (February 2015). “Single- and multiple-dose pharmacokinetics of samidorphan, a novel opioid antagonist, in healthy volunteers”. Clinical Therapeutics. 37 (2): 338–48. doi:10.1016/j.clinthera.2014.10.001. PMID 25456560.
- ^ Wentland MP, Lu Q, Lou R, Bu Y, Knapp BI, Bidlack, JM (April 2005). “Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone”. Bioorganic & Medicinal Chemistry Letters. 15 (8): 2107–10. doi:10.1016/j.bmcl.2005.02.032. PMID 15808478.
- ^ “Samidorphan”. Adis Insight. Springer Nature Switzerland AG.
- ^ Hillemacher T, Heberlein A, Muschler MA, Bleich S, Frieling H (August 2011). “Opioid modulators for alcohol dependence”. Expert Opinion on Investigational Drugs. 20 (8): 1073–86. doi:10.1517/13543784.2011.592139. PMID 21651459.
- ^ Clinical trial number NCT01366001 for “ALK33BUP-101: Safety and Pharmacodynamic Effects of ALKS 33-BUP Administered Alone and When Co-administered With Cocaine” at ClinicalTrials.gov
- ^ “ALKS 5461 drug found to reduce depressive symptoms in Phase 1/2 study”.
- ^ “Investigational ALKS 5461 Channels ‘Opium Cure’ for Depression”.
- ^ LaMattina J (15 January 2013). “Will Alkermes’ Antipsychotic ALKS-3831 Become Another Tredaptive?”. Forbes.
- ^ Correll, Christoph U.; Newcomer, John W.; Silverman, Bernard; DiPetrillo, Lauren; Graham, Christine; Jiang, Ying; Du, Yangchun; Simmons, Adam; Hopkinson, Craig; McDonnell, David; Kahn, René S. (2020-08-14). “Effects of Olanzapine Combined With Samidorphan on Weight Gain in Schizophrenia: A 24-Week Phase 3 Study”. American Journal of Psychiatry. 177 (12): 1168–1178. doi:10.1176/appi.ajp.2020.19121279. ISSN 0002-953X.
- ^ “FDA Panel: Some Risk OK for Olanzapine Combo With Less Weight Gain”. http://www.medpagetoday.com. 2020-10-09. Retrieved 2021-01-23.
- ^ Jump up to:a b Linda P. Dwoskin (29 January 2014). Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Elsevier Science. pp. 398–399, 402–403. ISBN 978-0-12-420177-4.
- ^ Jump up to:a b Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, et al. (April 2009). “Syntheses of novel high affinity ligands for opioid receptors”. Bioorganic & Medicinal Chemistry Letters. 19 (8): 2289–94. doi:10.1016/j.bmcl.2009.02.078. PMC 2791460. PMID 19282177.
- ^ McElroy SL, Guerdjikova AI, Blom TJ, Crow SJ, Memisoglu A, Silverman BL, Ehrich EW (April 2013). “A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder”. The International Journal of Eating Disorders. 46(3): 239–45. doi:10.1002/eat.22114. PMID 23381803.
External links
| Clinical data | |
|---|---|
| Other names | ALKS-33, RDC-0313; 3-Carboxamido-4-hydroxynaltrexone |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Elimination half-life | 7–9 hours[1] |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 852626-89-2 |
| PubChem CID | 11667832 |
| ChemSpider | 23259667 |
| UNII | 7W2581Z5L8 |
| KEGG | D10162 |
| Chemical and physical data | |
| Formula | C21H26N2O4 |
| Molar mass | 370.449 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI |
/////////samidorphan, サミドルファン, ALKS 33, ALKS-33, RDC-0313, RDC-0313-00, APPROVALS 2021, FDA 2021, Lybalvi
SMILESO[C@@H](CC(O)=O)C(O)=O.NC(=O)C1=CC=C2C[C@H]3N(CC4CC4)CC[C@@]4(CC(=O)CC[C@@]34O)C2=C1O
