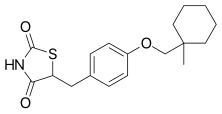
Ciglitazone
U-63287, ADD-3878
- Molecular FormulaC18H23NO3S
- Average mass333.445 Da
- 74772-77-3 [RN]
(±)-5-[4-(1-Methylcyclohexylmethoxy)benzyl]thiazolidine-2,4-dione2,4-Thiazolidinedione, 5-[[4-[(1-methylcyclohexyl)methoxy]phenyl]methyl]-5-[4-(1-methylcyclohexylmethoxy) benzyl]-thiazolidine-2,4-dione
Ciglitazone (INN) is a thiazolidinedione. Developed by Takeda Pharmaceuticals in the early 1980s, it is considered the prototypical compound for the thiazolidinedione class.[1][2][3][4]
Ciglitazone was never used as a medication, but it sparked interest in the effects of thiazolidinediones. Several analogues were later developed, some of which—such as pioglitazone and troglitazone—made it to the market.[2]
Ciglitazone significantly decreases VEGF production by human granulosa cells in an in vitro study, and may potentially be used in ovarian hyperstimulation syndrome.[5] Ciglitazone is a potent and selective PPARγ ligand. It binds to the PPARγ ligand-binding domain with an EC50 of 3.0 μM. Ciglitazone is active in vivo as an anti-hyperglycemic agent in the ob/ob murine model.[6] Inhibits HUVEC differentiation and angiogenesis and also stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells.[7]
SYN
T. Sohda, K. Mizuno, E. Imamiya, Y. Sugiyama, T. Fujita, and Y. Kawamatsu, Chem. Pharm. Bull., 30, 3580 (1982).

SYN
Ciglitazone (CAS NO.: ), with other name of , 5-((4-((1-methylcyclohexyl)methoxy)phenyl)methyl)-, (+-)-, could be produced through many synthetic methods.
Following is one of the reaction routes:

The reaction of 1-methylcyclohexylmethanol (II) with 4-chloronitrobenzene (III) by means of NaH in hot DMSO gives 4-(1-methylcyclohexylmethoxylnitrobenzene (III), which is reduced with H2 over Pd/C in methanol yielding 4-(1-methylcyclohexylmethoxylaniline (IV). Diazotation of (IV) with NaNO2 and HCl in water affords a solution of the corresponding diazonium chloride (V), which is condensed with methyl acrylate (VI) by means of Cu2O affording methyl 2-chloro-3-[4-(1-methylcyclohexylmethoxyl)phenyl]propionate (VII). The cyclization of (VII) with thiourea (VIII) by means of sodium acetate in hot 2-methoxyethanol gives 2-imino-5-[4-(1-methylcyclohexylmethoxy)benzyl]thiazolidin-4-one (IX), which is finally hydrolyzed with HCl in refluxing 2-methoxyethanol – water.
Syn
Chem Pharm Bull 1982,30(10),3580
The reaction of 1-methylcyclohexylmethanol (II) with 4-chloronitrobenzene (III) by means of NaH in hot DMSO gives 4-(1-methylcyclohexylmethoxylnitrobenzene (III), which is reduced with H2 over Pd/C in methanol yielding 4-(1-methylcyclohexylmethoxylaniline (IV). Diazotation of (IV) with NaNO2 and HCl in water affords a solution of the corresponding diazonium chloride (V), which is condensed with methyl acrylate (VI) by means of Cu2O affording methyl 2-chloro-3-[4-(1-methylcyclohexylmethoxyl)phenyl]propionate (VII). The cyclization of (VII) with thiourea (VIII) by means of sodium acetate in hot 2-methoxyethanol gives 2-imino-5-[4-(1-methylcyclohexylmethoxy)benzyl]thiazolidin-4-one (IX), which is finally hydrolyzed with HCl in refluxing 2-methoxyethanol – water.
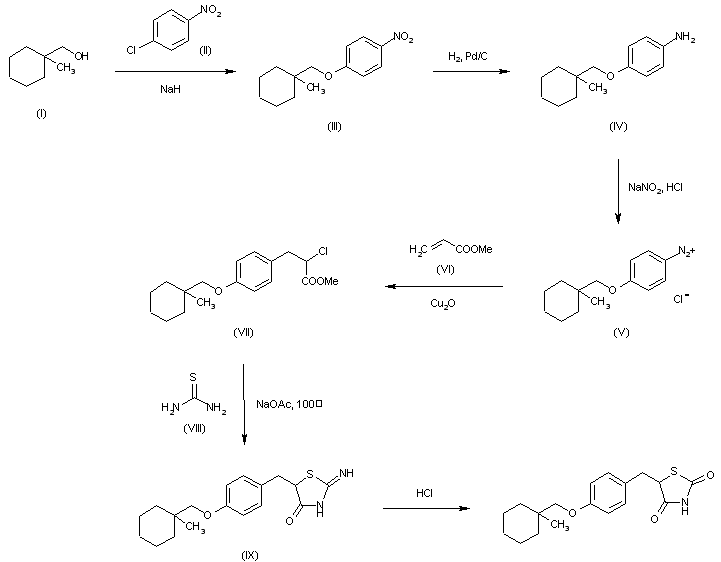
By cyclization of (VIII) with methyl 2-(methanesulfonyloxy)-3-[4-(1-methylcyclohexylmethoxy)phenyl]propionate (X) by means of sodium acetate in hot 2-methoxyethanol, followed by hydrolysis with HCl in ethanol water.

paper
Vijay Kumar Sharma , Anup Barde & Sunita Rattan (2020): A short review on synthetic strategies toward glitazone drugs, Synthetic Communications, DOI: 10.1080/00397911.2020.1821223
Experimental process for synthesis of ciglitazone is fairly robust, albeit pyrophorophic NaH as base is utilized for synthesis of 15.


Scheme 4. Reagents and conditions for the preparation of (R)-ciglitazone 24 (a) (S)-1-phenylethan-1- amine 19 (0.9 mol. equiv.), EtOH, RT, 4 h; (b) 1 N HCl (2 vol.), diethyl ether, RT, 10 min; (c) CH2N2 in diethyl ether (ca. 3% w/w), diethyl ether, 0 C-RT, 30 min; (d) KSCN (1.5 mol. equiv.), DMSO, 90 C, 2 h; (e) 2 N HCl (10 vol.), and EtOH, reflux 4 h.
Chiral synthesis Racemic-ciglitazone 17 was resolved with optically active a-methylbenzylamine (PEA) 19 through asymmetric transformation of optical lability at the C-5 position of TZD ring. 2-chloro-3-(4-((1-methylcyclohexyl)methoxy)phenyl)propanoic acid 18 was resolved using (S)-()-1-Phenylethylamine 19 to isolate (S)-2-chloro-3-(4-((1- methylcyclohexyl) methoxy)phenyl)propanoicacid 21. Esterification followed by substitution with KSCN provided methyl (R)-3-(4-((1-methylcyclohexyl)methoxy)phenyl)-2- thiocyanatopropan-oate 23 which was then hydrolyzed to isolate (R)-ciglitazone 24. Similarly, S-isomer was also isolated with (R)-(þ)-1-phenylethylamine (Scheme 4).
[30]Sohda, T.; Mizuno, K.; Kawamatsu, Y. Studies on Antidiabetic Agents. VI. Asymmetric Transformation of (þ/-)-5-[4-(1-Methylcyclohexylmethoxy)Benzyl]-2,4- Thiazolidinedione (Ciglitazone) with Optically Active 1-Phenylethylamines. Chem. Pharm. Bull. 1984, 32, 4460–4465. DOI: 10.1248/cpb.32.4460.
References
- ^ Pershadsingh HA, Szollosi J, Benson S, Hyun WC, Feuerstein BG, Kurtz TW (June 1993). “Effects of ciglitazone on blood pressure and intracellular calcium metabolism”. Hypertension. 21 (6 Pt 2): 1020–3. doi:10.1161/01.hyp.21.6.1020. PMID 8505086.
- ^ Jump up to:a b Hulin B, McCarthy PA, Gibbs EM (1996). “The glitazone family of antidiabetic agents”. Current Pharmaceutical Design. 2: 85–102.
- ^ Imoto H, Imamiya E, Momose Y, Sugiyama Y, Kimura H, Sohda T (October 2002). “Studies on non-thiazolidinedione antidiabetic agents. 1. Discovery of novel oxyiminoacetic acid derivatives”. Chem. Pharm. Bull. 50 (10): 1349–57. doi:10.1248/cpb.50.1349. PMID 12372861.
- ^ Sohda T, Kawamatsu Y, Fujita T, Meguro K, Ikeda H (November 2002). “[Discovery and development of a new insulin sensitizing agent, pioglitazone]”. Yakugaku Zasshi (in Japanese). 122 (11): 909–18. doi:10.1248/yakushi.122.909. PMID 12440149.
- ^ Shah DK, Menon KM, Cabrera LM, Vahratian A, Kavoussi SK, Lebovic DI (April 2010). “Thiazolidinediones decrease vascular endothelial growth factor (VEGF) production by human luteinized granulosa cells in vitro”. Fertil. Steril. 93 (6): 2042–7. doi:10.1016/j.fertnstert.2009.02.059. PMC 2847675. PMID 19342033.
- ^ Willson, T.M.; Cobb, J.E.; Cowan, D.J.; et al. (1996). “The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones”. J Med Chem. 39 (3): 665–668. doi:10.1021/jm950395a. PMID 8576907.
- ^ Xin, X.; et al. (1999). “Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo;”. J. Biol. Chem. 274 (13): 9116–21. doi:10.1074/jbc.274.13.9116. PMID 10085162.
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| IUPAC name[show] | |
| CAS Number | 74772-77-3 |
| PubChem CID | 2750 |
| IUPHAR/BPS | 2711 |
| DrugBank | DB09201 |
| ChemSpider | 2648 |
| UNII | U8QXS1WU8G |
| KEGG | D03493 |
| ChEMBL | ChEMBL7002 |
| CompTox Dashboard (EPA) | DTXSID0040757 |
| ECHA InfoCard | 100.220.474 |
| Chemical and physical data | |
| Formula | C18H23NO3S |
| Molar mass | 333.45 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES[hide]O=C1NC(=O)SC1Cc3ccc(OCC2(C)CCCCC2)cc3 | |
| InChI[hide]InChI=1S/C18H23NO3S/c1-18(9-3-2-4-10-18)12-22-14-7-5-13(6-8-14)11-15-16(20)19-17(21)23-15/h5-8,15H,2-4,9-12H2,1H3,(H,19,20,21) Key:YZFWTZACSRHJQD-UHFFFAOYSA-N |
/////////ciglitazone, U 63287, ADD 3878, DIABETES