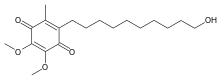
IDEBENONE
2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methyl-1,4-benzoquinone
- Molecular FormulaC19H30O5
- Average mass338.439 Da
- 58186-27-9
- Idebenona, Idebenonum, CV 2619
IdesolKS-5193NemocebralSNT-MC17идебенонإيديبينون艾地苯醌
Puldysa (idebenone), for the treatment of Duchenne muscular dystrophyTitle: Idebenone
CAS Registry Number: 58186-27-9
CAS Name: 2-(10-Hydroxydecyl)-5,6-dimethoxy-3-methyl-2,5-cyclohexadiene-1,4-dione
Additional Names: 6-(10-hydroxydecyl)-2,3-dimethoxy-5-methyl-1,4-benzoquinone; 2,3-dimethoxy-5-methyl-6-(10¢-hydroxydecyl)-1,4-benzoquinone; 6-(10-hydroxydecyl)ubiquinone
Manufacturers’ Codes: CV-2619
Trademarks: Avan (Takeda); Daruma (Takeda); Lucebanol (Hormona); Mnesis (Takeda)
Molecular Formula: C19H30O5Molecular Weight: 338.44
Percent Composition: C 67.43%, H 8.93%, O 23.64%
Literature References: Ubiquinone derivative with protective effects against cerebral ischemia. Prepn: H. Morimoto et al.,DE2519730; eidem,US4271083 (1975, 1981 both to Takeda); K. Okamoto et al.,Chem. Pharm. Bull.30, 2797 (1982); C.-A. Yu, L. Yu, Biochemistry21, 4096 (1982). Effect on ischemia-induced amnesia in rats: N. Yamazaki et al.,Jpn. J. Pharmacol.36, 349 (1984). Metabolism in animals: T. Kobayashi et al.,J. Pharmacobio-Dyn.8, 448 (1985). Disposition: H. Torii et al.,ibid. 457. Pharmacokinetics and tolerance in humans: M. F. Barkworth et al.,Arzneim.-Forsch.35, 1704 (1985). Series of articles on pharmacology and clinical studies: Arch. Gerontol. Geriatr.8, 193-366 (1989). Review of chemistry, toxicology and pharmacology: I. Zs-Nagy, Arch. Gerontol. Geriatr.11, 177-186 (1990).Properties: Orange needles from ligroin, mp 46-50° (Morimoto); also reported as crystals from hexane + ethyl acetate, mp 52-53° (Okamoto). Sol in organic solvents. Practically insol in water.Melting point: mp 46-50° (Morimoto); mp 52-53° (Okamoto)Therap-Cat: Nootropic.Keywords: Nootropic.
Idebenone is a member of the class of 1,4-benzoquinones which is substituted by methoxy groups at positions 2 and 3, by a methyl group at positions 5, and by a 10-hydroxydecyl group at positions 6. Initially developed for the treatment of Alzheimer’s disease, benefits were modest; it was subsequently found to be of benefit for the symptomatic treatment of Friedreich’s ataxia. It has a role as an antioxidant. It is a primary alcohol and a member of 1,4-benzoquinones.
Idebenone (pronounced eye-deb-eh-known, trade names Catena, Raxone, Sovrima, among others) is a drug that was initially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer’s disease and other cognitive defects.[1] This has been met with limited success. The Swiss company Santhera Pharmaceuticals has started to investigate it for the treatment of neuromuscular diseases. In 2010, early clinical trials for the treatment of Friedreich’s ataxia[2] and Duchenne muscular dystrophy[3] have been completed. As of December 2013 the drug is not approved for these indications in North America or Europe. It is approved by the European Medicines Agency (EMA) for use in Leber’s hereditary optic neuropathy (LHON) and was designated an orphan drug in 2007.[4]
Chemically, idebenone is an organic compound of the quinone family. It is also promoted commercially as a synthetic analog of coenzyme Q10 (CoQ10).
Uses
Indications that are or were approved in some territories
Nootropic effects and Alzheimer’s disease
Idebenone improved learning and memory in experiments with mice.[5] In humans, evaluation of Surrogate endpoints like electroretinography, auditory evoked potentials and visual analogue scales also suggested positive nootropic effects,[6] but larger studies with hard endpoints are missing.
Research on idebenone as a potential therapy of Alzheimer’s disease have been inconsistent, but there may be a trend for a slight benefit.[7][8] In May 1998, the approval for this indication was cancelled in Japan due to the lack of proven effects. In some European countries, the drug is available for the treatment of individual patients in special cases.[1]
Friedreich’s ataxia (Sovrima)
Preliminary testing has been done in humans and found idebenone to be a safe treatment for Friedreich’s ataxia (FA), exhibiting a positive effect on cardiac hypertrophy and neurological function.[9] The latter was only significantly improved in young patients.[10] In a different experiment, a one-year test on eight patients, idebenone reduced the rate of deterioration of cardiac function, but without halting the progression of ataxia.[11]
The drug was approved for FA in Canada in 2008 under conditions including proof of efficacy in further clinical trials.[12] However, on February 27, 2013, Health Canada announced that idebenone would be voluntarily recalled as of April 30, 2013 by its Canadian manufacturer, Santhera Pharmaceuticals, due to the failure of the drug to show efficacy in the further clinical trials that were conducted.[13] In 2008, the European Medicines Agency (EMA) refused a marketing authorisation for this indication.[1] As of 2013 the drug was not approved for FA in Europe[14] nor in the US, where there is no approved treatment.[15]
Leber’s hereditary optic neuropathy (Raxone)
Leber’s hereditary optic neuropathy (LHON) is a mitochondrially inherited (mother to all offspring) degeneration of retinal ganglion cells (RGCs) and their axons that leads to an acute or subacute loss of central vision; this affects predominantly young adult males. Santhera completed a Phase III clinical trial in this indication in Europe with positive results,[16] and submitted an application to market the drug to European regulators in July 2011.[17] It is approved by EMA for this indication and was designated an orphan drug in 2007.[4]
Indications being explored
Duchenne muscular dystrophy (Catena)
After experiments in mice[18] and preliminary studies in humans, idebenone has entered Phase II clinical trials in 2005[3] and Phase III trials in 2009.[19]
Other neuromuscular diseases
Phase I and II clinical trials for the treatment of MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes)[20] and primary progressive multiple sclerosis[21] are ongoing as of December 2013.
Life style
Idebenone is claimed to have properties similar to CoQ10 in its antioxidant properties, and has therefore been used in anti-aging on the basis of free-radical theory. Clinical evidence for this use is missing. It has been used in topical applications to treat wrinkles.[22]
Pharmacology
In cellular and tissue models, idebenone acts as a transporter in the electron transport chain of mitochondria and thus increases the production of adenosine triphosphate (ATP) which is the main energy source for cells, and also inhibits lipoperoxide formation. Positive effects on the energy household of mitochondria has also been observed in animal models.[1][23] Clinical relevance of these findings has not been established.
Pharmacokinetics
Idebenone is well absorbed from the gut but undergoes excessive first pass metabolism in the liver, so that less than 1% reach the circulation. This rate can be improved with special formulations (suspensions) of idebenone and by administering it together with fat food; but even taking these measures bioavailability still seems to be considerably less than 14% in humans. More than 99% of the circulating drug are bound to plasma proteins. Idebenone metabolites include glucuronides and sulfates, which are mainly (~80%) excreted via the urine.[1]
SYN
https://www.sciencedirect.com/science/article/abs/pii/S0040402014014306


SYN
The palladium-catalyzed olefination of a sp2 or benzylic carbon attached to a (pseudo)halogen is known as the Heck reaction.2,63 It is a powerful tool, mainly used for the synthesis of vinylarenes, and it has also been employed for the construction of conjugated double bonds. The widespread application of this reaction can be illustrated by numerous examples in both academia small-scale64 and industrial syntheses.5 As an example, in 2011, a idebenone (124) total synthesis based on a Heck reaction was described (Scheme 35).65 This compound, initially designed for the treatment of Alzheimer’s and Parkinson’s diseases, presented a plethora of other interesting activities, such as free radical scavenging and action against some muscular illnesses. The key step in the synthesis was the coupling of 2-bromo-3,4,5-trimethoxy-1-methylbenzene (125) with dec-9-en-1-ol affording products 126. Under non-optimized conditions (Pd(OAc)2, PPh3, Et3N, 120 ºC), a mixture composed of 60% linear olefins 126 and 15% of the undesired branched product 127 was obtained after three days of reaction. Therefore, the conditions were optimized, allowing the preparation of 126 in 67% yield with no detection of 127 after only 30 min of reaction employing DMF, Pd(PPh3)4, iPr2NEt under microwave heating. To conclude the synthesis, the Heck adducts were submitted to hydroxyl protection/deprotection, hydrogenation, and ring oxidation. After these reactions, idebenone was obtained with 20% overall yield over 6 steps.

Scheme 35 Synthesis of idebenone (124) based on Heck reaction of 2-bromo-3,4,5-trimethoxy-1-methylbenzene with dec-9-en-1-ol under microwave irradiation.
Syn
- Duveau, Damien Y.; Bioorganic & Medicinal Chemistry 2010, V18(17), P6429-6441
- Okada, Taiiti; EP 289223 A1 1988
- Watanabe, Masazumi; EP 58057 A1 1982
- Okamoto, Kayoko; Chemical & Pharmaceutical Bulletin 1982, V30(8), P2797-819
- “Drugs – Synonyms and Properties” data were obtained from Ashgate Publishing Co. (US)
Paper
Tsoukala, Anna; Organic Process Research & Development 2011, V15(3), P673-680
https://pubs.acs.org/doi/10.1021/op200051v
An environmentally benign, convenient, high yielding, and cost-effective synthesis leading to idebenone is disclosed. The synthesis includes a bromination process for the preparation of 2-bromo-3,4,5-trimethoxy-1-methylbenzene, a protocol for the Heck cross-coupling reaction using either thermal or microwave heating, olefin reduction by palladium catalyzed hydrogenation, and a green oxidation protocol with hydrogen peroxide as oxidant to achieve the benzoquinone framework. The total synthesis is composed of six steps that provide an overall yield of 20% that corresponds to a step yield of 76%.





PAPER
Bioorganic & Medicinal Chemistry 2010, V18(17), P6429-6441
https://www.sciencedirect.com/science/article/abs/pii/S0968089610006322

Analogues of mitoQ and idebenone were synthesized to define the structural elements that support oxygen consumption in the mitochondrial respiratory chain. Eight analogues were prepared and fully characterized, then evaluated for their ability to support oxygen consumption in the mitochondrial respiratory chain. While oxygen consumption was strongly inhibited by mitoQ analogues 2–4 in a chain length-dependent manner, modification of idebenone by replacement of the quinone methoxy groups by methyl groups (analogues 6–8) reduced, but did not eliminate, oxygen consumption. Idebenone analogues 6–8 also displayed significant cytoprotective properties toward cultured mammalian cells in which glutathione had been depleted by treatment with diethyl maleate.
Idebenone (5)18 To a stirred solution containing 200 mg (0.467 mmol) of 2,3- dimethoxy-6-methyl-5-benzyloxydecyl-p-benzoquinone (38) in 5 mL of anhydrous methanol at 23 C was added 15 mg of 10 % Pd/C in one portion. The reaction mixture was stirred at 23 C under an atmosphere of hydrogen for 24 h. Air was then bubbled through the reaction mixture at 23 C for 24 h. The suspension was filtered through Celite and the filtrate was concentrated under diminished pressure to afford idebenone (5) as an orange solid: yield 130 mg (82%); mp: 46–47 C; 1 H NMR (400 MHz, CDCl3) d 1.34 (m, 14H), 1.60 (quint, 2H, J = 7.6 Hz), 2.04 (s, 3H), 2.44 (t, 2H, J = 8.0 Hz), 3.63 (t, 2H, J = 6.8 Hz), and 3.99 (s, 6H); 13C NMR (100 MHz, CDCl3) d 11.9, 25.7, 26.4, 28.7, 29.3, 29.3, 29.4, 29.5, 29.8, 32.7
References
- ^ Jump up to:a b c d e “CHMP Assessment Report for Sovrima” (PDF). European Medicines Agency. 20 November 2008: 6, 9–11, 67f.
- ^ Clinical trial number NCT00229632 for “Idebenone to Treat Friedreich’s Ataxia” at ClinicalTrials.gov
- ^ Jump up to:a b Clinical trial number NCT00654784 for “Efficacy and Tolerability of Idebenone in Boys With Cardiac Dysfunction Associated With Duchenne Muscular Dystrophy (DELPHI)” at ClinicalTrials.gov
- ^ Jump up to:a b “Raxone”. http://www.ema.europa.eu. Retrieved 12 July 2019.
- ^ Liu, XJ; Wu, WT (1999). “Effects of ligustrazine, tanshinone II A, ubiquinone, and idebenone on mouse water maze performance”. Zhongguo Yao Li Xue Bao. 20 (11): 987–90. PMID 11270979.
- ^ Schaffler, K; Hadler, D; Stark, M (1998). “Dose-effect relationship of idebenone in an experimental cerebral deficit model. Pilot study in healthy young volunteers with piracetam as reference drug”. Arzneimittel-Forschung. 48 (7): 720–6. PMID 9706371.
- ^ Gutzmann, H; Kühl, KP; Hadler, D; Rapp, MA (2002). “Safety and efficacy of idebenone versus tacrine in patients with Alzheimer’s disease: results of a randomized, double-blind, parallel-group multicenter study”. Pharmacopsychiatry. 35 (1): 12–8. doi:10.1055/s-2002-19833. PMID 11819153.
- ^ Parnetti, L; Senin, U; Mecocci, P (1997). “Cognitive enhancement therapy for Alzheimer’s disease. The way forward”. Drugs. 53 (5): 752–68. doi:10.2165/00003495-199753050-00003. PMID 9129864. S2CID 46987059.
- ^ Di Prospero NA, Baker A, Jeffries N, Fischbeck KH (October 2007). “Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: a randomised, placebo-controlled trial”. Lancet Neurol. 6 (10): 878–86. doi:10.1016/S1474-4422(07)70220-X. PMID 17826341. S2CID 24749816.
- ^ Tonon C, Lodi R (September 2008). “Idebenone in Friedreich’s ataxia”. Expert Opin Pharmacother. 9 (13): 2327–37. doi:10.1517/14656566.9.13.2327. PMID 18710357. S2CID 73285881.
- ^ Buyse G, Mertens L, Di Salvo G, et al. (May 2003). “Idebenone treatment in Friedreich’s ataxia: neurological, cardiac, and biochemical monitoring”. Neurology. 60 (10): 1679–81. doi:10.1212/01.wnl.0000068549.52812.0f. PMID 12771265. S2CID 36556782.
- ^ “Heath Canada Fact Sheet – Catena”. Archived from the original on 19 June 2014.
- ^ Voluntary Withdrawal of Catena from the Canadian Market
- ^ Margaret Wahl for Quest Magazine, MAY 28, 2010. FA Research: Idebenone Strikes Out Again
- ^ NINDS Fact Sheet
- ^ Klopstock, T; et al. (2011). “A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy”. Brain. 134 (9): 2677–86. doi:10.1093/brain/awr170. PMC 3170530. PMID 21788663.
- ^ Staff (26 July 2011). “Santhera publishes pivotal trial results of idebenone and goes for EU approval”. European Biotechnology News. Archived from the original on 2013-02-17.
- ^ Buyse, GM; Van Der Mieren, G; Erb, M; D’hooge, J; Herijgers, P; Verbeken, E; Jara, A; Van Den Bergh, A; et al. (2009). “Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performance”. European Heart Journal. 30 (1): 116–24. doi:10.1093/eurheartj/ehn406. PMC 2639086. PMID 18784063.
- ^ Clinical trial number NCT01027884 for “Phase III Study of Idebenone in Duchenne Muscular Dystrophy (DMD) (DELOS)” at ClinicalTrials.gov
- ^ Clinical trial number NCT00887562 for “Study of Idebenone in the Treatment of Mitochondrial Encephalopathy Lactic Acidosis & Stroke-like Episodes (MELAS)” at ClinicalTrials.gov
- ^ Clinical trial number NCT00950248 for “Double Blind Placebo-Controlled Phase I/II Clinical Trial of Idebenone in Patients With Primary Progressive Multiple Sclerosis (IPPoMS)” at ClinicalTrials.gov
- ^ McDaniel D, Neudecker B, Dinardo J, Lewis J, Maibach H (September 2005). “Clinical efficacy assessment in photodamaged skin of 0.5% and 1.0% idebenone”. J Cosmet Dermatol. 4 (3): 167–73. doi:10.1111/j.1473-2165.2005.00305.x. PMID 17129261. S2CID 2394666.
- ^ Suno M, Nagaoka A (May 1988). “[Effect of idebenone and various nootropic drugs on lipid peroxidation in rat brain homogenate in the presence of succinate]”. Nippon Yakurigaku Zasshi (in Japanese). 91 (5): 295–9. doi:10.1254/fpj.91.295. PMID 3410376.
| Clinical data | |
|---|---|
| Trade names | Catena, Raxone, Sovrima |
| AHFS/Drugs.com | International Drug Names |
| License data | EU EMA: by INN |
| ATC code | N06BX13 (WHO) |
| Legal status | |
| Legal status | In general: ℞ (Prescription only) |
| Pharmacokinetic data | |
| Bioavailability | <1% (high first pass effect) |
| Protein binding | >99% |
| Elimination half-life | 18 hours |
| Excretion | Urine (80%) and feces |
| Identifiers | |
| IUPAC name[show] | |
| CAS Number | 58186-27-9 |
| PubChem CID | 3686 |
| ChemSpider | 3558 |
| UNII | HB6PN45W4J |
| KEGG | D01750 |
| ChEMBL | ChEMBL252556 |
| CompTox Dashboard (EPA) | DTXSID0040678 |
| Chemical and physical data | |
| Formula | C19H30O5 |
| Molar mass | 338.444 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES[hide]O=C1/C(=C(\C(=O)C(\OC)=C1\OC)C)CCCCCCCCCCO | |
| InChI[hide]InChI=1S/C19H30O5/c1-14-15(12-10-8-6-4-5-7-9-11-13-20)17(22)19(24-3)18(23-2)16(14)21/h20H,4-13H2,1-3H3 Key:JGPMMRGNQUBGND-UHFFFAOYSA-N |
////////////IDEBENONE, Puldysa, Duchenne muscular dystrophy, Idesol, KS 5193, Nemocebral, SNT MC17, идебенон, إيديبينون , 艾地苯醌 , CV 2619
CC1=C(C(=O)C(=C(C1=O)OC)OC)CCCCCCCCCCO