

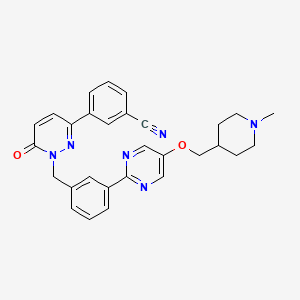
Tepotinib hydrochloride
CS-977;Tepotinib;Veledimex;MSC2156119;EMD-1214063
3-[1-[[3-[5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl]phenyl]methyl]-6-oxopyridazin-3-yl]benzonitrile;hydrate;hydrochloride
Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo-3-pyridazinyl)-, hydrochloride, hydrate
3- (1- {3- [5- (1-methylpiperidin-4-ylmethoxy) pyrimidine) -2-yl] -benzyl} -6-oxo-1,6-dihydro-pyridazin-3-yl) -benzonitrileтепотиниб [Russian] [INN]تيبوتينيب [Arabic] [INN]特泊替尼 [Chinese] [INN]
- 3-[1,6-Dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]benzonitrile
- 3-{1-[(3-{5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin2-yl}phenyl)methyl]-6-oxo-1,6-dihydropyridazin-3-yl}benzonitrile
- EMD 1214063
- MSC 2156119
| Formula | C29H28N6O2. HCl. H2OC29H28N6O2FREE |
|---|---|
| CAS | 1946826-82-9 HCL.H2OCAS No. FREE 1100598-32-0 |
| Mol weight | 547.0478492.57 FREE |
JAPAN 25/3 2020 APPROVED, Tepmetko
| Antineoplastic, Receptor tyrosine kinase inhibitor |

SYN

Bioorganic & Medicinal Chemistry Letters, 25(7), 1597-1602; 2015
PATENT
WO 2009006959
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009006959
Example 40
The preparation of the compound 3- (1- {3- [5- (1-Methyl-piperidin-4-ylmethoxy) -pyrimidin-2-yl] -benzyl} -6-oxo-1,6-dihydro-pyridazin-3 -yl) -benzonitrile (“A257”) takes place analogously to the following scheme
40.1 17.7 g (67.8 mmol) triphenyl are added to a suspension of 13.0 g (56.5 mmol) 3- (5-hydroxypyrimidin-2-yl) -benzoic acid methyl ester and 13.4 g (62.1 mmol) N-Boc-piperidinemethanol in 115 ml THF -phosphine and cooled to 5 ° C. To the suspension kept at this temperature, 13.3 ml (67.8 mmol) of diisopropylazodicarboxylate are added dropwise with stirring within 45 minutes. The reaction mixture is stirred for 1 hour at room temperature. Then a further 22.2 g (84.7 mmol) triphenylphosphine and 16.6 ml (84.7 mmol)
Diisopropyl azodicarboxylate added. The reaction mixture turns 18
Stirred for hours at room temperature and concentrated in vacuo. The resulting solid is filtered off with suction, washed with diethyl ether and chromatographed on a silica gel column with dichloromethane / methanol as the mobile phase: 4- [2- (3-methoxycarbonyl-phenyl) -pyrimidin-5-yloxymethyl] -piperidine-1-carboxylic acid tert .-butyl ester as lemon yellow crystals;
166 ° C .; ESI 428.
40.2 To a suspension of 1.71 g (3.99 mmol) of 4- [2- (3-methoxycarbonyl-phenyl) -pyrimidin-5-yloxymethyl] -piperidine-1-carboxylic acid tert-butyl ester in 20 ml of THF are added under nitrogen 25 ml (25 mmol) of a 1 M solution of diisobutylaluminum hydride in THF were added dropwise. The reaction mixture is stirred at room temperature for 1 hour, and 1 ml of a saturated sodium sulfate solution is added. The resulting precipitate is filtered off with suction and washed with THF and hot 2-propanol. The filtrate is evaporated and recrystallized from tert-butyl methyl ether: {3- [5- (1-Methyl-piperidin-4-ylmethoxy) -pyrimidin-2-yl] -phenyl} -methanol as beige crystals; Mp 175 ° C; ESI 314.
40.3 To a solution of 313 mg (1.00 mmol) {3- [5- (1-methyl-piperidin-4-ylmethoxy) -pyrimidin-2-yl] -phenyl} -methanol in 2 ml THF are successively added 264 mg (1.30 mmol) 3- (6-oxo-1, 6-dihydro-pyridazin-3-yl) benzonitrile and 397 mg (1.5 mmol) triphenylphosphine are added. The reaction mixture is cooled in an ice bath and
294 μl (1.5 mmol) of diisopropylazodicarboxylate are added dropwise with stirring. The
The reaction mixture is stirred for 18 hours at room temperature and evaporated. The residue is chromatographed on a silica gel column using dichloromethane / methanol. The product-containing fractions are combined, evaporated, the residue digested with tert-butyl methyl ether, filtered off with suction and dried in vacuo: 3- (1- {3- [5- (1-methylpiperidin-4-ylmethoxy) pyrimidine) -2-yl] -benzyl} -6-oxo-1,6-dihydro-pyridazin-3-yl) -benzonitrile as colorless crystals; M.p. 177 ° C; ESI 493;
1 H-NMR (de-DMSO): δ [ppm] = 1.33 (m, 2H), 1.75 (m, 3H), 1.89 (m, 2H), 2.17 (S, 3H), 2.80 (m, 2H), 4.05 (d, J = 6.1 Hz 1 2H), 5.45 (s, 2H) 1 7.16 (d, J = 10 Hz, 1 H), 7.49 (m, 2H), 7.73 (t, J = 7.8 Hz, 1H ), 7.93 (d, J = 7.8 Hz, 1H) 1 8.17 (d, J = 10 Hz, 1H), 8.24 (m, 2H), 8.38 (m, 2H), 8.64 (s, 2H).
The hemisulfate, citrate, tartrate, sulfate, succinate and hydrochloride are obtained from “A257” by salt formation.
PATENT
WO 2009007074
PAPER
Bioorganic & Medicinal Chemistry Letters (2015), 25(7), 1597-1602.
https://www.sciencedirect.com/science/article/abs/pii/S0960894X15000955
PAPER
Molecules (2019), 24(6), 1173/1-1173/16.
https://www.mdpi.com/1420-3049/24/6/1173

Scheme 1. Reagents and conditions: a) PdCl2(PPh3)2, Na2CO3, ethanol/toluene/water, 90 °C, 8 h; b) SOCl2, CHCl3, reflux; c) SeO2, dioxane:H2O = 10:1, reflux, 12 h; d) NaOH, −30 °C; e) NaH, DMF/THF, 0 °C—room temperature, 12 h; f) dry ethanol, reflux; g) NaOH, DMF/H2O, 60 °C, 8 h, N2.

Scheme 2. Reagents and conditions: a) N,N-diisopropylethylamine, dry CH2Cl2, 0 °C—room temperature, 6 h; b) PdCl2(PPh3)2, Na2CO3, ethanol/toluene/water, 90 °C, 8 h; c) 10% aq. HCl, MeOH, reflux; d) K2CO3, dry DMF, 80 °C, 12 h; e) NaOH, DMF/H2O, 60 °C, 8 h, N2; f) PPh3, DIAD, THF, 0 °C—room temperature; g) SOCl2, CHCl3, reflux; h) 35% formaldehyde, NaBH4, MeOH.

Scheme 3. Reagents and conditions: a) PdCl2(PPh3)2, Na2CO3, ethanol/toluene/water, 90 °C, 8 h; b) NaBH4, MeOH, 0 °C—room temperature, 1 h; c) SOCl2, CHCl3, reflux; d) K2CO3, dry DMF, 80 °C, 12 h; e) 31a–31b: NaOH, DMF/H2O, 60 °C, 8 h, N2; f) 31c–31g: NaH, dry DMF, 0 °C—room temperature, 5 h.

Scheme 4. Reagents and conditions: a) K2CO3, dry DMF, 80 °C, 12 h; b) PdCl2(PPh3)2, Na2CO3, DME/DMF/water, 89 °C, 12 h; c) NaOH, DMF/H2O, 60 °C, 8 h, N2.

Scheme 5. Reagents and conditions: a) K2CO3, dry DMF, 80 °C, 12 h; b) PdCl2(PPh3)2, Na2CO3, DME/DMF/water, 89 °C, 12 h; c) NaOH, DMF/H2O, 60 °C, 8 h, N2.
///////////Tepotinib, Tepotinib hydrochloride, Tepmetko, JAPAN 2020, 2020 APPROVALS, тепотиниб , تيبوتينيب , 特泊替尼 , EMD 1214063, MSC 2156119
CN1CCC(CC1)COC2=CN=C(N=C2)C3=CC=CC(=C3)CN4C(=O)C=CC(=N4)C5=CC=CC(=C5)C#N.O.Cl