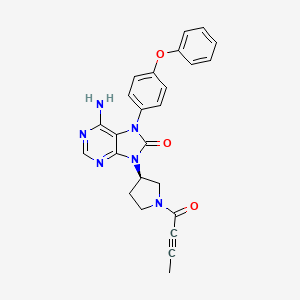
Tirabrutinib
チラブルチニブ塩酸塩
GS-4059
ONO-4059
6-amino-9-[(3R)-1-but-2-ynoylpyrrolidin-3-yl]-7-(4-phenoxyphenyl)purin-8-one
| Formula | C25H22N6O3. HCl |
|---|---|
| CAS | 1439901-97-9 HCL1351636-18-4FREE FORM |
| Mol weight | 490.9415 |
JAPAN APPROVED 2020/3/25 Velexbru
Antineoplastic, Bruton’s tyrosine kinase inhibitor
8H-Purin-8-one,6-amino-7,9-dihydro-9-((3R)-1-(1-oxo-2-butyn-1-yl)-3-pyrrolidinyl)-7-(4-phenoxyphenyl)
6-Amino-9-((3R)-1-(2-butynoyl)-3-pyrrolidinyl)-7-(4-phenoxyphenyl)-7,9-dihydro-8H-purin-8-one
Tirabrutinib (Velexbru®) is an orally administered, small molecule, Bruton’s tyrosine kinase (BTK) inhibitor being developed by Ono Pharmaceutical and its licensee Gilead Sciences for the treatment of autoimmune disorders and haematological malignancies. Tirabrutinib irreversibly and covalently binds to BTK in B cells and inhibits aberrant B cell receptor signalling in B cell-related cancers and autoimmune diseases. In March 2020, oral tirabrutinib was approved in Japan for the treatment of recurrent or refractory primary central nervous system lymphoma. Tirabrutinib is also under regulatory review in Japan for the treatment of Waldenström’s macroglobulinemia and lymphoplasmacytic lymphoma. Clinical development is underway in the USA, Europe and Japan for autoimmune disorders, chronic lymphocytic leukaemia, B cell lymphoma, Sjogren’s syndrome, pemphigus and rheumatoid arthritis. This article summarizes the milestones in the development of tirabrutinib leading to the first approval of tirabrutinib for the treatment of recurrent or refractory primary central nervous system lymphoma in Japan.

PATENT
WO 2011152351
https://patents.google.com/patent/WO2011152351A1/en
Example 19 (2) : 6-amino-9-[(3R) -1- (2-butinoyl) -3-pyrrolidinyl] -7- (4-phenoxyphenyl) -7,9-dihydro-8H-purine- 8-on

TLC: Rf 0.68 (ethyl acetate: methanol = 9: 1);
1 H-NMR (CDCl 3 ): δ 1.94-2.03, 2.23-2.39, 2.80-3.01, 3.50-3.63, 3.67-3.80, 3.86-4.02, 4.03-4.18, 4.23-4.33, 4.42-4.51, 5.11-5.25, 7.04-7.23, 7.34-7.45, 8.20-8.23.
PATENT
WO 2013081016
WO 2015193740
WO 2015181633
WO 2015185998
WO 2016024228
WO 2016024231
WO 2016163531
WO 2016024227
WO 2017033113
PATENT
US 20170035881
https://patents.google.com/patent/US20170035881A1/en
PATENT WO 2017033113
https://patents.google.com/patent/WO2017033113A1/en

///////Tirabrutinib, japan 2020, 2020 approvals, Velexbru , チラブルチニブ塩酸塩 , GS 4059, ONO 4059,
CC#CC(=O)N1CCC(C1)N2C3=NC=NC(=C3N(C2=O)C4=CC=C(C=C4)OC5=CC=CC=C5)N


















