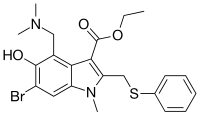
Umifenovir
- Molecular FormulaC22H25BrN2O3S
- Average mass477.414 Da
Umifenovir[2] (trade names Arbidol Russian: Арбидол, Chinese: 阿比朵尔) is an antiviral treatment for influenza infection used in Russia[3] and China. The drug is manufactured by Pharmstandard (Russian: Фармстандарт). Although some Russian studies have shown it to be effective, it is not approved for use in other countries. It is not approved by FDA for the treatment or prevention of influenza.[4] Chemically, umifenovir features an indole core, functionalized at all but one positions with different substituents. The drug is claimed to inhibit viral entry into target cells and stimulate the immune response. Interest in the drug has been renewed as a result of the SARS-CoV-2 outbreak.
Umifenovir is manufactured and made available as tablets, capsules and syrup.
Status
Testing of umifenovir’s efficacy has mainly occurred in China and Russia,[5][6] and it is well known in these two countries.[7] Some of the Russian tests showed the drug to be effective[5] and a direct comparison with Tamiflu showed similar efficiency in vitro and in a clinical setting.[8] In 2007, Arbidol (umifenovir) had the highest sales in Russia among all over-the-counter drugs.
Mode of action
Biochemistry
Umifenovir inhibits membrane fusion.[3] Umifenovir prevents contact between the virus and target host cells. Fusion between the viral envelope (surrounding the viral capsid) and the cell membrane of the target cell is inhibited. This prevents viral entry to the target cell, and therefore protects it from infection.[9]
Some evidence suggests that the drug’s actions are more effective at preventing infections from RNA viruses than infections from DNA viruses.[10]
As well as specific antiviral action against both influenza A and influenza B viruses, umifenovir exhibits modulatory effects on the immune system. The drug stimulates a humoral immune response, induces interferon-production, and stimulates the phagocytic function of macrophages.[11]
Clinical application
Umifenovir is used primarily as an antiviral treatments for influenza. The drug has also been investigated as a candidate drug for treatment of hepatitis C.[12]
More recent studies indicate that umifenovir also has in vitro effectiveness at preventing entry of Ebolavirus Zaïre Kikwit, Tacaribe arenavirus and human herpes virus 8 in mammalian cell cultures, while confirming umifenovir’s suppressive effect in vitro on Hepatitis B and poliovirus infection of mammalian cells when introduced either in advance of viral infection or during infection.[13][14]
Research
In February 2020, Li Lanjuan, an expert of the National Health Commission of China, proposed using Arbidol (umifenovir) together with darunavir as a potential treatment during the 2019–20 coronavirus pandemic.[15] Chinese experts claim that preliminary tests had shown that arbidol and darunavir could inhibit replication of the virus.[16][17] So far without additional effect if added on top of recombinant human interferon α2b spray.[18]
Side effects
Side effects in children include sensitization to the drug. No known overdose cases have been reported and allergic reactions are limited to people with hypersensitivity. The LD50 is more than 4 g/kg.[19]
Criticism
In 2007, the Russian Academy of Medical Sciences stated that the effects of Arbidol (umifenovir) are not scientifically proven.[20]
Russian media criticized lobbying attempts by Tatyana Golikova (then-Minister of Healthcare) to promote umifenovir,[21] and the unproven claim that Arbidol can speed up recovery from flu or cold by 1.3-2.3 days.[22] They also debunked claims that the efficacy of umifenovir is supported by peer-reviewed studies.[23][24]
Clip
https://www.sciencedirect.com/science/article/pii/S0960894X1730687X

CLIP
1,2-Dimethyl-5-hydroxyindole-3-acetic acid ethyl ester (I) is acetylated with acetic anhydride affording the O-acyl derivative (II) , which is brominated to the corresponding dibromide compound (III) . The reaction of (III) with thiophenol in KOH yields (IV) , which is then submitted to a conventional Mannich condensation with formaldehyde and dimethylamine in acetic acid, giving the free base of arbidol (V), which is treated with aqueous hydrochloric acid .

References
- ^ “Full Prescribing Information: Arbidol® (umifenovir) film-coated tablets 50 and 100 mg: Corrections and Additions”. State Register of Medicines (in Russian). Open joint-stock company “Pharmstandard-Tomskchempharm”. Retrieved 3 June 2015.
- ^ Recommended INN: List 65., WHO Drug Information, Vol. 25, No. 1, 2011, page 91
- ^ Jump up to:a b Leneva IA, Russell RJ, Boriskin YS, Hay AJ (February 2009). “Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol”. Antiviral Research. 81 (2): 132–40. doi:10.1016/j.antiviral.2008.10.009. PMID 19028526.
- ^ “FDA Approved Drugs for Influenza”. U.S. Food and Drug Administration.
- ^ Jump up to:a b Leneva IA, Fediakina IT, Gus’kova TA, Glushkov RG (2005). “[Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A]”. Terapevticheskii Arkhiv (Russian translation). ИЗДАТЕЛЬСТВО “МЕДИЦИНА”. 77 (8): 84–8. PMID 16206613.
- ^ Wang MZ, Cai BQ, Li LY, Lin JT, Su N, Yu HX, Gao H, Zhao JZ, Liu L (June 2004). “[Efficacy and safety of arbidol in treatment of naturally acquired influenza]”. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Academiae Medicinae Sinicae. 26 (3): 289–93. PMID 15266832.
- ^ Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ (2008). “Arbidol: a broad-spectrum antiviral compound that blocks viral fusion”. Current Medicinal Chemistry. 15 (10): 997–1005. doi:10.2174/092986708784049658. PMID 18393857.
- ^ Leneva IA, Burtseva EI, Yatsyshina SB, Fedyakina IT, Kirillova ES, Selkova EP, Osipova E, Maleev VV (February 2016). “Virus susceptibility and clinical effectiveness of anti-influenza drugs during the 2010-2011 influenza season in Russia”. International Journal of Infectious Diseases. 43: 77–84. doi:10.1016/j.ijid.2016.01.001. PMID 26775570.
- ^ Boriskin YS, Pécheur EI, Polyak SJ (July 2006). “Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection”. Virology Journal. 3: 56. doi:10.1186/1743-422X-3-56. PMC 1559594. PMID 16854226.
- ^ Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, Hou W, Cheng L, Xiao H, Yang Z (2007). “Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo”. Archives of Virology. 152 (8): 1447–55. doi:10.1007/s00705-007-0974-5. PMID 17497238.
- ^ Glushkov RG, Gus’kova TA, Krylova LIu, Nikolaeva IS (1999). “[Mechanisms of arbidole’s immunomodulating action]”. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk (in Russian) (3): 36–40. PMID 10222830.
- ^ Pécheur EI, Lavillette D, Alcaras F, Molle J, Boriskin YS, Roberts M, Cosset FL, Polyak SJ (May 2007). “Biochemical mechanism of hepatitis C virus inhibition by the broad-spectrum antiviral arbidol”. Biochemistry. 46 (20): 6050–9. doi:10.1021/bi700181j. PMC 2532706. PMID 17455911.
- ^ Pécheur EI, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW, Polyak SJ (January 2016). “The Synthetic Antiviral Drug Arbidol Inhibits Globally Prevalent Pathogenic Viruses”. Journal of Virology. 90 (6): 3086–92. doi:10.1128/JVI.02077-15. PMC 4810626. PMID 26739045.
- ^ Hulseberg CE, Fénéant L, Szymańska-de Wijs KM, Kessler NP, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ, White JM. Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses. J Virol. 2019 Apr 3;93(8). pii: e02185-18. doi:10.1128/JVI.02185-18 PMID 30700611
- ^ Ng E (4 February 2020). “Coronavirus: are cocktail therapies for flu and HIV the magic cure?”. South China Morning Post.
Bangkok and Hangzhou hospitals put combination remedies to the test.
- ^ Zheng W, Lau M (4 February 2020). “China’s health officials say priority is to stop mild coronavirus cases from getting worse”. South China Morning Post.
- ^ Lu H (January 2020). “Drug treatment options for the 2019-new coronavirus (2019-nCoV)”. Bioscience Trends. doi:10.5582/bst.2020.01020. PMID 31996494.
- ^ “Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia”. 4 February 2020. Retrieved 24 February 2020.
- ^ “АРБИДОЛ® (ARBIDOL)”. Vidal. Archived from the originalon 4 February 2009.
- ^ “Resolution”. Meetings of the Presidium of the Formulary Committee. Russian Academy of Medical Sciences. 16 March 2007.
- ^ “How do we plant federal ministers”. MKRU. 21 April 2011.
- ^ Golunov I (23 December 2013). “13 most popular flu cures: do they work?”. Professional Journalism Platform.
- ^ Reuters S. “Stick in the wheel”. Esquire.
- ^ “Repetition – the mother of learning”. Esquire.
External links
- “Мастерлек” Pharmaceuticals, Moscow, Russia. Patent number No. 2033157, Registry number No. 003610/01.
- (in Russian) Arbidol
- English published clinical studies and translations for Arbidol 1973–2016
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Arbidol |
| Pregnancy category |
|
| Routes of administration |
Oral (hard capsules, tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 17–21 hours |
| Excretion | 40% excrete as unchanged umifenovir in feces (38.9%) and urine (0.12%)[1] |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.800 |
| Chemical and physical data | |
| Formula | C22H25BrN2O3S |
| Molar mass | 477.41 g/mol g·mol−1 |
| 3D model (JSmol) | |
Umifenovir is an indole-based, hydrophobic, dual-acting direct antiviral/host-targeting agent used for the treatment and prophylaxis of influenza and other respiratory infections.13 It has been in use in Russia for approximately 25 years and in China since 2006. Its invention is credited to a collaboration between Russian scientists from several research institutes 40-50 years ago, and reports of its chemical synthesis date back to 1993.13 Umifenovir’s ability to exert antiviral effects through multiple pathways has resulted in considerable investigation into its use for a variety of enveloped and non-enveloped RNA and DNA viruses, including Flavivirus,2 Zika virus,3 foot-and-mouth disease,4 Lassa virus,6 Ebola virus,6 herpes simplex,8, hepatitis B and C viruses, chikungunya virus, reovirus, Hantaan virus, and coxsackie virus B5.13,9 This dual activity may also confer additional protection against viral resistance, as the development of resistance to umifenovir does not appear to be significant.13
Umifenovir is currently being investigated as a potential treatment and prophylactic agent for COVID-19 caused by SARS-CoV2 infections in combination with both currently available and investigational HIV therapies.1,16,17
Indication
Umifenovir is currently licensed in China and Russia for the prophylaxis and treatment of influenza and other respiratory viral infections.13 It has demonstrated activity against a number of viruses and has been investigated in the treatment of Flavivirus,2 Zika virus,3 foot-and-mouth disease,4 Lassa virus,6 Ebola virus,6 and herpes simplex.8 In addition, it has shown in vitro activity against hepatitis B and C viruses, chikungunya virus, reovirus, Hantaan virus, and coxsackie virus B5.13,9
Umifenovir is currently being investigated as a potential treatment and prophylactic agent for the prevention of COVID-19 caused by SARS-CoV-2 infections.1,16
Pharmacodynamics
Umifenovir exerts its antiviral effects via both direct-acting virucidal activity and by inhibiting one (or several) stage(s) of the viral life cycle.13 Its broad-spectrum of activity covers both enveloped and non-enveloped RNA and DNA viruses. It is relatively well-tolerated and possesses a large therapeutic window – weight-based doses up to 100-fold greater than those used in humans failed to produce any pathological changes in test animals.13
Umifenovir does not appear to result in significant viral resistance. Instances of umifenovir-resistant influenza virus demonstrated a single mutation in the HA2 subunit of influenza hemagglutinin, suggesting resistance is conferred by prevention of umifenovir’s activity related to membrane fusion. The mechanism through which other viruses may become resistant to umifenovir requires further study.13
Mechanism of action
Umifenovir is considered both a direct-acting antiviral (DAA) due to direct virucidal effects and a host-targeting agent (HTA) due to effects on one or multiple stages of viral life cycle (e.g. attachment, internalization), and its broad-spectrum antiviral activity is thought to be due to this dual activity.13 It is a hydrophobic molecule capable of forming aromatic stacking interactions with certain amino acid residues (e.g. tyrosine, tryptophan), which contributes to its ability to directly act against viruses. Antiviral activity may also be due to interactions with aromatic residues within the viral glycoproteins involved in fusion and cellular recognition,5,7 with the plasma membrane to interfere with clathrin-mediated exocytosis and intracellular trafficking,10 or directly with the viral lipid envelope itself (in enveloped viruses).13,12 Interactions at the plasma membrane may also serve to stabilize it and prevent viral entry (e.g. stabilizing influenza hemagglutinin inhibits the fusion step necessary for viral entry).13
Due to umifenovir’s ability to interact with both viral proteins and lipids, it may also interfere with later stages of the viral life cycle. Some virus families, such as Flaviviridae, replicate in a subcellular compartment called the membranous web – this web requires lipid-protein interactions that may be hindered by umifenovir. Similarly, viral assembly of hepatitis C viruses is contingent upon the assembly of lipoproteins, presenting another potential target.13
Absorption
Umifenovir is rapidly absorbed following oral administration, with an estimated Tmax between 0.65-1.8 hours.14,15,13 The Cmax has been estimated as 415 – 467 ng/mL and appears to increase linearly with dose,14,15 and the AUC0-inf following oral administration has been estimated to be approximately 2200 ng/mL/h.14,15
Volume of distribution
Data regarding the volume of distribution of umifenovir are currently unavailable.
Protein binding
Data regarding protein-binding of umifenovir are currently unavailable.
Metabolism
Umifenovir is highly metabolized in the body, primarily in hepatic and intestinal microsomess, with approximately 33 metabolites having been observed in human plasma, urine, and feces.14 The principal phase I metabolic pathways include sulfoxidation, N-demethylation, and hydroxylation, followed by phase II sulfate and glucuronide conjugation. In the urine, the major metabolites were sulfate and glucuronide conjugates, while the major species in the feces was unchanged parent drug (~40%) and the M10 metabolite (~3.0%). In the plasma, the principal metabolites are M6-1, M5, and M8 – of these, M6-1 appears of most importance given its high plasma exposure and long elimination half-life (~25h), making it a potentially important player in the safety and efficacy of umifenovir.14
Enzymes involved in the metabolism of umifenovir include members of the cytochrome P450 family (primarily CYP3A4), flavin-containing monooxygenase (FMO) family, and UDP-glucuronosyltransferase (UGT) family (specifically UGT1A9 and UGT2B7).14,11
- Lu H: Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020 Jan 28. doi: 10.5582/bst.2020.01020. [PubMed:31996494]
- Haviernik J, Stefanik M, Fojtikova M, Kali S, Tordo N, Rudolf I, Hubalek Z, Eyer L, Ruzek D: Arbidol (Umifenovir): A Broad-Spectrum Antiviral Drug That Inhibits Medically Important Arthropod-Borne Flaviviruses. Viruses. 2018 Apr 10;10(4). pii: v10040184. doi: 10.3390/v10040184. [PubMed:29642580]
- Fink SL, Vojtech L, Wagoner J, Slivinski NSJ, Jackson KJ, Wang R, Khadka S, Luthra P, Basler CF, Polyak SJ: The Antiviral Drug Arbidol Inhibits Zika Virus. Sci Rep. 2018 Jun 12;8(1):8989. doi: 10.1038/s41598-018-27224-4. [PubMed:29895962]
- Herod MR, Adeyemi OO, Ward J, Bentley K, Harris M, Stonehouse NJ, Polyak SJ: The broad-spectrum antiviral drug arbidol inhibits foot-and-mouth disease virus genome replication. J Gen Virol. 2019 Sep;100(9):1293-1302. doi: 10.1099/jgv.0.001283. Epub 2019 Jun 4. [PubMed:31162013]
- Kadam RU, Wilson IA: Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):206-214. doi: 10.1073/pnas.1617020114. Epub 2016 Dec 21. [PubMed:28003465]
- Hulseberg CE, Feneant L, Szymanska-de Wijs KM, Kessler NP, Nelson EA, Shoemaker CJ, Schmaljohn CS, Polyak SJ, White JM: Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses. J Virol. 2019 Apr 3;93(8). pii: JVI.02185-18. doi: 10.1128/JVI.02185-18. Print 2019 Apr 15. [PubMed:30700611]
- Zeng LY, Yang J, Liu S: Investigational hemagglutinin-targeted influenza virus inhibitors. Expert Opin Investig Drugs. 2017 Jan;26(1):63-73. doi: 10.1080/13543784.2017.1269170. Epub 2016 Dec 14. [PubMed:27918208]
- Li MK, Liu YY, Wei F, Shen MX, Zhong Y, Li S, Chen LJ, Ma N, Liu BY, Mao YD, Li N, Hou W, Xiong HR, Yang ZQ: Antiviral activity of arbidol hydrochloride against herpes simplex virus I in vitro and in vivo. Int J Antimicrob Agents. 2018 Jan;51(1):98-106. doi: 10.1016/j.ijantimicag.2017.09.001. Epub 2017 Sep 7. [PubMed:28890393]
- Pecheur EI, Borisevich V, Halfmann P, Morrey JD, Smee DF, Prichard M, Mire CE, Kawaoka Y, Geisbert TW, Polyak SJ: The Synthetic Antiviral Drug Arbidol Inhibits Globally Prevalent Pathogenic Viruses. J Virol. 2016 Jan 6;90(6):3086-92. doi: 10.1128/JVI.02077-15. [PubMed:26739045]
- Blaising J, Levy PL, Polyak SJ, Stanifer M, Boulant S, Pecheur EI: Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking. Antiviral Res. 2013 Oct;100(1):215-9. doi: 10.1016/j.antiviral.2013.08.008. Epub 2013 Aug 25. [PubMed:23981392]
- Song JH, Fang ZZ, Zhu LL, Cao YF, Hu CM, Ge GB, Zhao DW: Glucuronidation of the broad-spectrum antiviral drug arbidol by UGT isoforms. J Pharm Pharmacol. 2013 Apr;65(4):521-7. doi: 10.1111/jphp.12014. Epub 2012 Dec 24. [PubMed:23488780]
- Teissier E, Zandomeneghi G, Loquet A, Lavillette D, Lavergne JP, Montserret R, Cosset FL, Bockmann A, Meier BH, Penin F, Pecheur EI: Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS One. 2011 Jan 25;6(1):e15874. doi: 10.1371/journal.pone.0015874. [PubMed:21283579]
- Blaising J, Polyak SJ, Pecheur EI: Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014 Jul;107:84-94. doi: 10.1016/j.antiviral.2014.04.006. Epub 2014 Apr 24. [PubMed:24769245]
- Deng P, Zhong D, Yu K, Zhang Y, Wang T, Chen X: Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob Agents Chemother. 2013 Apr;57(4):1743-55. doi: 10.1128/AAC.02282-12. Epub 2013 Jan 28. [PubMed:23357765]
- Liu MY, Wang S, Yao WF, Wu HZ, Meng SN, Wei MJ: Pharmacokinetic properties and bioequivalence of two formulations of arbidol: an open-label, single-dose, randomized-sequence, two-period crossover study in healthy Chinese male volunteers. Clin Ther. 2009 Apr;31(4):784-92. doi: 10.1016/j.clinthera.2009.04.016. [PubMed:19446151]
- Wang Z, Chen X, Lu Y, Chen F, Zhang W: Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020 Feb 9. doi: 10.5582/bst.2020.01030. [PubMed:32037389]
- Nature Biotechnology: Coronavirus puts drug repurposing on the fast track [Link]
/////////////////Arbidol, umifenovir, covid 19, corona virus, Арбидол , 阿比朵尔 ,
CCOC(=O)C1=C(CSC2=CC=CC=C2)N(C)C2=CC(Br)=C(O)C(CN(C)C)=C12

https://eurekalert.org/pub_releases/2020-02/nuos-edm022620.php
.jpg)

