
| Chemical Formula: | C5H4FN3O2 | |
| CAS #: | 259793-96-9 | |
| Molecular Weight: | 157.1 | |
ANTI-INFLUENZA COMPOUND |
||
| clinical trials | http://clinicaltrials.gov/search/intervention=Favipiravir | |
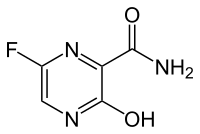
| Chemical Name: | 6-fluoro-3-hydroxy-2-pyrazinecarboxamide | |
| Synonyms: | T-705, T705, Favipiravir |
- Molecular FormulaC5H4FN3O2
- Average mass157.103 Da
6-Fluoro-3-hydroxypyrazine-2-carboxamide
C5H4FN3O2 : 157.1
[259793-96-9]
https://www.pmda.go.jp/files/000210319.pdf
The drug substance is a white to light yellow powder. It is sparingly soluble in acetonitrile and in methanol, and slightly soluble in water and in ethanol (99.5). It is slightly soluble at pH 2.0 to 5.5 and sparingly soluble at pH 5.5 to 6.1. The drug substance is not hygroscopic at 25°C/51% to 93%RH. The melting point is 187°C to 193°C, and the dissociation constant (pKa) is 5.1 due to the hydroxyl group of favipiravir. Measurement results on the partition ratio of favipiravir in water/octanol at 25°C indicate that favipiravir tends to be distributed in the 1-octanol phase at pH 2 to 4 and in the water phase at pH 5 to 13.
Any batch manufactured by the current manufacturing process is in Form A. The stability study does not show any change in crystal form over time; and a change from Form A to Form B is unlikely.
Experimental Properties
| PROPERTY | VALUE | SOURCE |
|---|---|---|
| melting point (°C) | 187℃ to 193℃ | https://www.pmda.go.jp/files/000210319.pdf |
| water solubility | slightly soluble in water | https://www.pmda.go.jp/files/000210319.pdf |
| pKa | 5.1 | https://www.pmda.go.jp/files/000210319.pdf |
Favipiravir, also known as T-705, Avigan, or favilavir is an antiviral drug being developed by Toyama Chemical (Fujifilm group) of Japan with activity against many RNA viruses. Like certain other experimental antiviral drugs (T-1105 and T-1106), it is a pyrazinecarboxamide derivative. In experiments conducted in animals Favipiravir has shown activity against influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses.[1]Activity against enteroviruses[2] and Rift Valley fever virus has also been demonstrated.[3] Favipiravir has showed limited efficacy against Zika virus in animal studies, but was less effective than other antivirals such as MK-608.[4] The agent has also shown some efficacy against rabies,[5] and has been used experimentally in some humans infected with the virus.[6]
In February 2020 Favipiravir was being studied in China for experimental treatment of the emergent COVID-19 (novel coronavirus)disease.[7][8] On March 17 Chinese officials suggested the drug had been effective in treating COVID in Wuhan and Shenzhen.[9][10]
Discovered by Toyama Chemical Co., Ltd. in Japan, favipiravir is a modified pyrazine analog that was initially approved for therapeutic use in resistant cases of influenza.7,9 The antiviral targets RNA-dependent RNA polymerase (RdRp) enzymes, which are necessary for the transcription and replication of viral genomes.7,12,13
Not only does favipiravir inhibit replication of influenza A and B, but the drug shows promise in the treatment of influenza strains that are resistant to neuramidase inhibitors, as well as avian influenza.9,19 Favipiravir has been investigated for the treatment of life-threatening pathogens such as Ebola virus, Lassa virus, and now COVID-19.10,14,15
Mechanism of action
The mechanism of its actions is thought to be related to the selective inhibition of viral RNA-dependent RNA polymerase.[11] Other research suggests that favipiravir induces lethal RNA transversion mutations, producing a nonviable viral phenotype.[12] Favipiravir is a prodrug that is metabolized to its active form, favipiravir-ribofuranosyl-5′-triphosphate (favipiravir-RTP), available in both oral and intravenous formulations.[13][14] Human hypoxanthine guanine phosphoribosyltransferase (HGPRT) is believed to play a key role in this activation process.[15] Favipiravir does not inhibit RNA or DNA synthesis in mammalian cells and is not toxic to them.[1] In 2014, favipiravir was approved in Japan for stockpiling against influenza pandemics.[16] However, favipiravir has not been shown to be effective in primary human airway cells, casting doubt on its efficacy in influenza treatment.[17]
Approval status
In 2014, Japan approved Favipiravir for treating viral strains unresponsive to current antivirals.[18]
In March 2015, the US Food and Drug Administration completed a Phase III clinical trial studying the safety and efficacy of Favipiravir in the treatment of influenza.[19]
Ebola virus trials
Some research has been done suggesting that in mouse models Favipiravir may have efficacy against Ebola. Its efficacy against Ebola in humans is unproven.[20][21][22] During the 2014 West Africa Ebola virus outbreak, it was reported that a French nurse who contracted Ebola while volunteering for MSF in Liberia recovered after receiving a course of favipiravir.[23] A clinical trial investigating the use of favipiravir against Ebola virus disease was started in Guéckédou, Guinea, during December 2014.[24] Preliminary results showed a decrease in mortality rate in patients with low-to-moderate levels of Ebola virus in the blood, but no effect on patients with high levels of the virus, a group at a higher risk of death.[25] The trial design has been criticised by Scott Hammer and others for using only historical controls.[26] The results of this clinical trial were presented in February 2016 at the annual Conference on Retroviruses and Opportunistic Infections (CROI) by Daouda Sissoko[27] and published on March 1, 2016 in PLOS Medicine.[28]
SARS-CoV-2 virus disease
In March 2020, Chinese officials suggested Favipiravir may be effective in treating COVID-19.[29]
SYN
https://link.springer.com/article/10.1007/s11696-018-0654-9


Electronic supplementary material
Below is the link to the electronic supplementary material.
Ref
https://pdfs.semanticscholar.org/be8e/cb882b99204983d2f60077c7ab8b53f4d62c.pdf
Drug Discoveries & Therapeutics. 2014; 8(3):117-120.
As a RNA polymerase inhibitor, 6-fluoro-3-hydroxypyrazine-2-carboxamide commercially named favipiravir has been proved to have potent inhibitory activity against RNA viruses in vitro and in vivo. A four-step synthesis of the compound is described in this article, amidation, nitrification, reduction and fluorination with an overall yield of about 8%. In addition, we reported the crystal structure of the title compound. The molecule is almost planar and the intramolecular O−H•••O hydrogen bond makes a 6-member ring. In the crystal, molecules are packing governed by both hydrogen bonds and stacking interactions.


2.2.1. Preparation of 3-hydroxypyrazine-2-carboxamide To a suspension of 3-hydroxypyrazine-2-carboxylic acid (1.4 g, 10 mmol) in 150 mL MeOH, SOCl2 was added dropwise at 40°C with magnetic stirring for 6 h resulting in a bright yellow solution. The reaction was then concentrated to dryness. The residue was dissolved in 50 mL 25% aqueous ammonia and stirred overnight to get a suspension. The precipitate was collected and dried. The solid yellow-brown crude product was recrystallization with 50 mL water to get the product as pale yellow crystals (1.1 g, 78%). mp = 263-265°C. 1 H-NMR (300 MHz, DMSO): δ 13.34 (brs, 1H, OH), 8.69 (s, 1H, pyrazine H), 7.93-8.11 (m, 3H, pyrazine H, CONH2). HRMS (ESI): m/z [M + H]+ calcd for C5H6N3O2 + : 140.0460; found: 140.0457.
2.2.2. Preparation of 3-hydroxy-6-nitropyrazine-2- carboxamide In the solution of 3-hydroxypyrazine-2-carboxamide (1.0 g, 7 mmol) in 6 mL concentrate sulfuric acid under ice-cooling, potassium nitrate (1.4 g, 14 mmol) was added. After stirring at 40°C for 4 h, the reaction mixture was poured into 60 mL water. The product was collected by fi ltration as yellow solid (0.62 g, 48%). mp = 250-252°C. 1 H-NMR (600 MHz, DMSO): δ 12.00- 15.00 (br, 1H, OH), 8.97 (s, 1H, pyrazine H), 8.32 (s, 1H, CONH2), 8.06 (s, 1H, CONH2). 13C-NMR (75 MHz, DMSO): δ 163.12, 156.49, 142.47, 138.20, 133.81. HRMS (ESI): m/z [M + H]+ calcd for C5H5N4O4 + : 185.0311; found: 185.0304.
2.2.3. Preparation of 6-amino-3-hydroxypyrazine-2- carboxamide 3-Hydroxy-6-nitropyrazine-2-carboxamide (0.6 g, 3.3 mmol) and a catalytic amount of raney nickel were suspended in MeOH, then hydrazine hydrate was added dropwise. The resulting solution was refl uxed 2 h, cooled, filtered with diatomite, and then MeOH is evaporated in vacuo to get the crude product as dark brown solid without further purification (0.4 g, 77%). HRMS (ESI): m/z [M + H]+ calcd for C5H7N4O2 + : 155.0569; found:155.0509.
2.2.4. Preparation of 6-fluoro-3-hydroxypyrazine-2- carboxamide To a solution of 6-amino-3-hydroxypyrazine-2- carboxamide (0.4 g, 2.6 mmol) in 3 mL 70% HFpyridine aqueous at -20°C under nitrogen atmosphere, sodium nitrate (0.35 g, 5.2 mmol) was added. After stirring 20 min, the solution was warmed to room temperature for another one hour. Then 20 mL ethyl acetate/water (1:1) were added, after separation of the upper layer, the aqueous phase is extracted with four 20 mL portions of ethyl acetate. The combined extracts are dried with anhydrous magnesium sulfate and concentrated to dryness to get crude product as oil. The crude product was purified by chromatography column as white solid (0.12 g, 30%). mp = 178-180°C. 1 H-NMR (600 MHz, DMSO): δ 12.34 (brs, 1H, OH), 8.31 (d, 1H, pyrazine H, J = 8.0 Hz), 7.44 (s, 1H, CONH2), 5.92 (s, 1H, CONH2). 13C-NMR (75 MHz, DMSO): δ 168.66, 159.69, 153.98, 150.76, 135.68. HRMS (ESI): m/z [M + H]+ calcd for C5H5FN3O2 + : 158.0366; found: 158.0360.
SEE
Chemical Papers (2019), 73(5), 1043-1051.
PAPER
Medicinal chemistry (Shariqah (United Arab Emirates)) (2018), 14(6), 595-603
http://www.eurekaselect.com/158990/article
PATENT
CN 107641106
PAPER
Chemical Papers (2017), 71(11), 2153-2158.
https://link.springer.com/article/10.1007%2Fs11696-017-0208-6



Below is the link to the electronic supplementary material.
References
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D. F.; Barnard, D. L.; Gowen, B. B.; Julander, J. G.; Morrey, J. D. (2009). “T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections”. Antiviral Research 82 (3): 95–102. doi:10.1016/j.antiviral.2009.02.198. PMID 19428599. edit
![]()
- WO 2000010569
- WO 2008099874
- WO 201009504
- WO 2010104170
- WO 2012063931





CLIP
Influenza virus is a central virus of the cold syndrome, which has attacked human being periodically to cause many deaths amounting to tens millions. Although the number of deaths shows a tendency of decrease in the recent years owing to the improvement in hygienic and nutritive conditions, the prevalence of influenza is repeated every year, and it is apprehended that a new virus may appear to cause a wider prevalence.
For prevention of influenza virus, vaccine is used widely, in addition to which low molecular weight substances such as Amantadine and Ribavirin are also used
CLIP

Synthesis of Favipiravir
ZHANG Tao1, KONG Lingjin1, LI Zongtao1,YUAN Hongyu1, XU Wenfang2*
(1. Shandong Qidu PharmaceuticalCo., Ltd., Linzi 255400; 2. School of Pharmacy, Shandong University, Jinan250012)
ABSTRACT: Favipiravir was synthesized from3-amino-2-pyrazinecarboxylic acid by esterification, bromination with NBS,diazotization and amination to give 6-bromo-3-hydroxypyrazine-2-carboxamide,which was subjected to chlorination with POCl3, fluorination with KF, andhydrolysis with an overall yield of about 22%.
PATENT
US6787544

| subs G1 | G2 | G3 | G4 | R2 |
| compd 32 N | CH | C—CF3 | N | H |
…………………
EP2192117

Example 1-1
To a 17.5 ml N,N-dimethylformamide solution of 5.0 g of 3,6-difluoro-2-pyrazinecarbonitrile, a 3.8 ml water solution of 7.83 g of potassium acetate was added dropwise at 25 to 35° C., and the solution was stirred at the same temperature for 2 hours. 0.38 ml of ammonia water was added to the reaction mixture, and then 15 ml of water and 0.38 g of active carbon were added. The insolubles were filtered off and the filter cake was washed with 11 ml of water. The filtrate and the washing were joined, the pH of this solution was adjusted to 9.4 with ammonia water, and 15 ml of acetone and 7.5 ml of toluene were added. Then 7.71 g of dicyclohexylamine was added dropwise and the solution was stirred at 20 to 30° C. for 45 minutes. Then 15 ml of water was added dropwise, the solution was cooled to 10° C., and the precipitate was filtered and collected to give 9.44 g of dicyclohexylamine salt of 6-fluoro-3-hydroxy-2-pyradinecarbonitrile as a lightly yellowish white solid product.
1H-NMR (DMSO-d6) δ values: 1.00-1.36 (10H, m), 1.56-1.67 (2H, m), 1.67-1.81 (4H, m), 1.91-2.07 (4H, m), 3.01-3.18 (2H, m), 8.03-8.06 (1H, m), 8.18-8.89 (1H, broad)
Example 1-2
4.11 ml of acetic acid was added at 5 to 15° C. to a 17.5 ml N,N-dimethylformamide solution of 5.0 g of 3,6-difluoro-2-pyrazinecarbonitrile. Then 7.27 g of triethylamine was added dropwise and the solution was stirred for 2 hours. 3.8 ml of water and 0.38 ml of ammonia water were added to the reaction mixture, and then 15 ml of water and 0.38 g of active carbon were added. The insolubles were filtered off and the filter cake was washed with 11 ml of water. The filtrate and the washing were joined, the pH of the joined solution was adjusted to 9.2 with ammonia water, and 15 ml of acetone and 7.5 ml of toluene were added to the solution, followed by dropwise addition of 7.71 g of dicyclohexylamine. Then 15 ml of water was added dropwise, the solution was cooled to 5° C., and the precipitate was filtered and collected to give 9.68 g of dicyclohexylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile as a slightly yellowish white solid product.
Examples 2 to 5
The compounds shown in Table 1 were obtained in the same way as in Example 1-1.
| TABLE 1 | |||
 |
|||
| Example No. | Organic amine | Example No. | Organic amine |
| 2 | Dipropylamine | 4 | Dibenzylamine |
| 3 | Dibutylamine | 5 | N-benzylmethylamine |
Dipropylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) 6 values: 0.39 (6H, t, J=7.5 Hz), 1.10 (4H, sex, J=7.5 Hz), 2.30-2.38 (4H, m), 7.54 (1H, d, J=8.3 Hz)
Dibutylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) 6 values: 0.36 (6H, t, J=7.3 Hz), 0.81 (4H, sex, J=7.3 Hz), 0.99-1.10 (4H, m), 2.32-2.41 (4H, m), 7.53 (1H, d, J=8.3 Hz)
Dibenzylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) δ values: 4.17 (4H, s), 7.34-7.56 (10H, m), 8.07 (1H, d, J=8.3 Hz)
N-benzylmethylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) δ values: 2.57 (3H, s), 4.14 (2H, s), 7.37-7.53 (5H, m), 8.02-8.08 (1H, m)
Preparation Example 1
300 ml of toluene was added to a 600 ml water solution of 37.5 g of sodium hydroxide. Then 150 g of dicyclohexylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile was added at 15 to 25° C. and the solution was stirred at the same temperature for 30 minutes. The water layer was separated and washed with toluene, and then 150 ml of water was added, followed by dropwise addition of 106 g of a 30% hydrogen peroxide solution at 15 to 30° C. and one-hour stirring at 20 to 30° C. Then 39 ml of hydrochloric acid was added, the seed crystals were added at 40 to 50° C., and 39 ml of hydrochloric acid was further added dropwise at the same temperature. The solution was cooled to 10° C. the precipitate was filtered and collected to give 65.6 g of 6-fluoro-3-hydroxy-2-pyrazinecarboxamide as a slightly yellowish white solid.
1H-NMR (DMSO-d6) δ values: 8.50 (1H, s), 8.51 (1H, d, J=7.8 Hz), 8.75 (1H, s), 13.41 (1H, s)
CLIP
jan 2014
First patient enrolled in the North American Phase 3 clinical trials for investigational flu treatment drug
Eur J Med Chem. 2013 Apr;62:534-44. doi: 10.1016/j.ejmech.2013.01.015. Epub 2013 Jan 29.
| US3631036 * | Nov 4, 1969 | Dec 28, 1971 | American Home Prod | 5-amino-2 6-substituted-7h-pyrrolo(2 3-d) pyrimidines and related compounds |
| US3745161 * | Apr 20, 1970 | Jul 10, 1973 | Merck & Co Inc | Phenyl-hydroxy-pyrazine carboxylic acids and derivatives |
| US4404203 * | May 14, 1981 | Sep 13, 1983 | Warner-Lambert Company | Substituted 6-phenyl-3(2H)-pyridazinones useful as cardiotonic agents |
| US4545810 * | Mar 25, 1983 | Oct 8, 1985 | Sds Biotech Corporation | Herbicidal and plant growth regulant diphenylpyridazinones |
| US4565814 * | Jan 18, 1984 | Jan 21, 1986 | Sanofi | Pyridazine derivatives having a psychotropic action and compositions |
| US4661145 * | Sep 20, 1984 | Apr 28, 1987 | Rohm And Haas Company | Plant growth regulating 1-aryl-1,4-dihydro-4-oxo(thio)-pyridazines |
| US5420130 | May 16, 1994 | May 30, 1995 | Synthelabo | 2-aminopyrazine-5-carboxamide derivatives, their preparation and their application in therapeutics |
| US5459142 * | Aug 23, 1993 | Oct 17, 1995 | Otsuka Pharmaceutical Co., Ltd. | Pyrazinyl and piperazinyl substituted pyrazine compounds |
| US5597823 | Jun 5, 1995 | Jan 28, 1997 | Abbott Laboratories | Tricyclic substituted hexahydrobenz [e]isoindole alpha-1 adrenergic antagonists |
| US6159980 * | Sep 15, 1997 | Dec 12, 2000 | Dupont Pharmaceuticals Company | Pyrazinones and triazinones and their derivatives thereof |
| EP0023358A1 * | Jul 28, 1980 | Feb 4, 1981 | Rohm And Haas Company | Process for the preparation of pyridazine derivatives |
| GB1198688A | Title not available | |||
| HU9401512A | Title not available | |||
| JPH09216883A * | Title not available | |||
| JPS5620576A | Title not available |
- ^ Jump up to:a b Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD (June 2009). “T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections”. Antiviral Research. 82 (3): 95–102. doi:10.1016/j.antiviral.2009.02.198. PMID 19428599.
- ^ Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (November 2013). “Favipiravir (T-705), a novel viral RNA polymerase inhibitor”. Antiviral Research. 100 (2): 446–54. doi:10.1016/j.antiviral.2013.09.015. PMC 3880838. PMID 24084488.
- ^ Caroline AL, Powell DS, Bethel LM, Oury TD, Reed DS, Hartman AL (April 2014). “Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever”. PLoS Neglected Tropical Diseases. 8 (4): e2790. doi:10.1371/journal.pntd.0002790. PMC 3983105. PMID 24722586.
- ^ Mumtaz N, van Kampen JJ, Reusken CB, Boucher CA, Koopmans MP (2016). “Zika Virus: Where Is the Treatment?”. Current Treatment Options in Infectious Diseases. 8 (3): 208–211. doi:10.1007/s40506-016-0083-7. PMC 4969322. PMID 27547128.
- ^ Yamada K, Noguchi K, Komeno T, Furuta Y, Nishizono A (April 2016). “Efficacy of Favipiravir (T-705) in Rabies Postexposure Prophylaxis”. The Journal of Infectious Diseases. 213 (8): 1253–61. doi:10.1093/infdis/jiv586. PMC 4799667. PMID 26655300.
- ^ Murphy J, Sifri CD, Pruitt R, Hornberger M, Bonds D, Blanton J, Ellison J, Cagnina RE, Enfield KB, Shiferaw M, Gigante C, Condori E, Gruszynski K, Wallace RM (January 2019). “Human Rabies – Virginia, 2017”. MMWR. Morbidity and Mortality Weekly Report. 67(5152): 1410–1414. doi:10.15585/mmwr.mm675152a2. PMC 6334827. PMID 30605446.
- ^ Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nature Reviews Drug Discovery 2020 Feb doi:10.1038/d41573-020-00016-0
- ^ BRIEF-Corrected-Zhejiang Hisun Pharma gets approval for clinical trial to test flu drug Favipiravir for pneumonia caused by new coronavirus. Reuters Healthcare, February 16, 2020.
- ^ [1]NHK World News ‘China: Avigan effective in tackling coronavirus’
- ^ Huaxia. Favipiravir shows good clinical efficacy in treating COVID-19: official. Xinhuanet.com, 17 March 2020
- ^ Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J (2013). “The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5′-triphosphate towards influenza A virus polymerase”. PLOS ONE. 8 (7): e68347. Bibcode:2013PLoSO…868347J. doi:10.1371/journal.pone.0068347. PMC 3707847. PMID 23874596.
- ^ Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA (April 2013). “T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro”. Journal of Virology. 87 (7): 3741–51. doi:10.1128/JVI.02346-12. PMC 3624194. PMID 23325689.
- ^ Guedj J, Piorkowski G, Jacquot F, Madelain V, Nguyen TH, Rodallec A, et al. (March 2018). “Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques”. PLoS Medicine. 15 (3): e1002535. doi:10.1371/journal.pmed.1002535. PMC 5870946. PMID 29584730.
- ^ Smee DF, Hurst BL, Egawa H, Takahashi K, Kadota T, Furuta Y (October 2009). “Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells”. The Journal of Antimicrobial Chemotherapy. 64 (4): 741–6. doi:10.1093/jac/dkp274. PMC 2740635. PMID 19643775.
- ^ Naesens L, Guddat LW, Keough DT, van Kuilenburg AB, Meijer J, Vande Voorde J, Balzarini J (October 2013). “Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir)”. Molecular Pharmacology. 84 (4): 615–29. doi:10.1124/mol.113.087247. PMID 23907213.
- ^ Koons C (7 August 2014). “Ebola Drug From Japan May Emerge Among Key Candidates”. Bloomberg.com.
- ^ Yoon JJ, Toots M, Lee S, Lee ME, Ludeke B, Luczo JM, et al. (August 2018). “Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses”. Antimicrobial Agents and Chemotherapy. 62 (8): e00766–18. doi:10.1128/AAC.00766-18. PMC 6105843. PMID 29891600.
- ^ Hayden, Frederick. “Influenza virus polymerase inhibitors in clinical development”. Current Opinion in Infectious Diseases. doi:10.1097/QCO.0000000000000532.
- ^ “Phase 3 Efficacy and Safety Study of Favipiravir for Treatment of Uncomplicated Influenza in Adults – T705US316”. FDA. Retrieved 17 March 2020.
- ^ Gatherer D (August 2014). “The 2014 Ebola virus disease outbreak in West Africa”. The Journal of General Virology. 95 (Pt 8): 1619–24. doi:10.1099/vir.0.067199-0. PMID 24795448.
- ^ Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S (May 2014). “Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model”. Antiviral Research. 105: 17–21. doi:10.1016/j.antiviral.2014.02.014. PMID 24583123.
- ^ Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS (April 2014). “Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model”. Antiviral Research. 104: 153–5. doi:10.1016/j.antiviral.2014.01.012. PMID 24462697.
- ^ “First French Ebola patient leaves hospital”. Reuters. 4 October 2016.
- ^ “Guinea: Clinical Trial for Potential Ebola Treatment Started in MSF Clinic in Guinea”. AllAfrica – All the Time. Retrieved 28 December 2014.
- ^ Fink S (4 February 2015). “Ebola Drug Aids Some in a Study in West Africa”. The New York Times.
- ^ Cohen J (26 February 2015). “Results from encouraging Ebola trial scrutinized”. Science. doi:10.1126/science.aaa7912. Retrieved 21 January 2016.
- ^ “Favipiravir in Patients with Ebola Virus Disease: Early Results of the JIKI trial in Guinea | CROI Conference”. croiconference.org. Retrieved 2016-03-17.
- ^ Sissoko D, Laouenan C, Folkesson E, M’Lebing AB, Beavogui AH, Baize S, et al. (March 2016). “Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea”. PLoS Medicine. 13(3): e1001967. doi:10.1371/journal.pmed.1001967. PMC 4773183. PMID 26930627.
- ^ “Japanese flu drug ‘clearly effective’ in treating coronavirus, says China”. The Guardian. 2020-03-18. Retrieved 2020-03-18.\
- Beigel J, Bray M: Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. doi: 10.1016/j.antiviral.2008.01.003. Epub 2008 Feb 4. [PubMed:18328578]
- Hsieh HP, Hsu JT: Strategies of development of antiviral agents directed against influenza virus replication. Curr Pharm Des. 2007;13(34):3531-42. [PubMed:18220789]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW: In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007 Sep;51(9):3168-76. Epub 2007 Jul 2. [PubMed:17606691]
- Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y: Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007 Mar;51(3):845-51. Epub 2006 Dec 28. [PubMed:17194832]
- Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K: Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005 Mar;49(3):981-6. [PubMed:15728892]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K: In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002 Apr;46(4):977-81. [PubMed:11897578]
- Furuta Y, Komeno T, Nakamura T: Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449-463. doi: 10.2183/pjab.93.027. [PubMed:28769016]
- Venkataraman S, Prasad BVLS, Selvarajan R: RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution. Viruses. 2018 Feb 10;10(2). pii: v10020076. doi: 10.3390/v10020076. [PubMed:29439438]
- Hayden FG, Shindo N: Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis. 2019 Apr;32(2):176-186. doi: 10.1097/QCO.0000000000000532. [PubMed:30724789]
- Madelain V, Nguyen TH, Olivo A, de Lamballerie X, Guedj J, Taburet AM, Mentre F: Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials. Clin Pharmacokinet. 2016 Aug;55(8):907-23. doi: 10.1007/s40262-015-0364-1. [PubMed:26798032]
- Nguyen TH, Guedj J, Anglaret X, Laouenan C, Madelain V, Taburet AM, Baize S, Sissoko D, Pastorino B, Rodallec A, Piorkowski G, Carazo S, Conde MN, Gala JL, Bore JA, Carbonnelle C, Jacquot F, Raoul H, Malvy D, de Lamballerie X, Mentre F: Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017 Feb 23;11(2):e0005389. doi: 10.1371/journal.pntd.0005389. eCollection 2017 Feb. [PubMed:28231247]
- de Farias ST, Dos Santos Junior AP, Rego TG, Jose MV: Origin and Evolution of RNA-Dependent RNA Polymerase. Front Genet. 2017 Sep 20;8:125. doi: 10.3389/fgene.2017.00125. eCollection 2017. [PubMed:28979293]
- Shu B, Gong P: Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation. Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):E4005-14. doi: 10.1073/pnas.1602591113. Epub 2016 Jun 23. [PubMed:27339134]
- Nagata T, Lefor AK, Hasegawa M, Ishii M: Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep. 2015 Feb;9(1):79-81. doi: 10.1017/dmp.2014.151. Epub 2014 Dec 29. [PubMed:25544306]
- Rosenke K, Feldmann H, Westover JB, Hanley PW, Martellaro C, Feldmann F, Saturday G, Lovaglio J, Scott DP, Furuta Y, Komeno T, Gowen BB, Safronetz D: Use of Favipiravir to Treat Lassa Virus Infection in Macaques. Emerg Infect Dis. 2018 Sep;24(9):1696-1699. doi: 10.3201/eid2409.180233. Epub 2018 Sep 17. [PubMed:29882740]
- Delang L, Abdelnabi R, Neyts J: Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018 May;153:85-94. doi: 10.1016/j.antiviral.2018.03.003. Epub 2018 Mar 7. [PubMed:29524445]
- Nature Biotechnology: Coronavirus puts drug repurposing on the fast track [Link]
- Pharmaceuticals and Medical Devices Agency: Avigan (favipiravir) Review Report [Link]
- World Health Organization: Influenza (Avian and other zoonotic) [Link]
 |
|
| Names | |
|---|---|
| IUPAC name
5-Fluoro-2-hydroxypyrazine-3-carboxamide
|
|
| Other names
T-705; Avigan; favilavir
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C5H4FN3O2 | |
| Molar mass | 157.104 g·mol−1 |
| Pharmacology | |
| J05AX27 (WHO) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
////////////FAVIPIRAVIR, ファビピラビル , 8916, Avigan, T-705, favilavir, COVID-19, coronavirus, antiinfluenza

Want to know everything on vir series
click
http://drugsynthesisint.blogspot.in/p/vir-series-hep-c-virus-22.html
AND
http://medcheminternational.blogspot.in/p/vir-series-hep-c-virus.html








