NIDUFEXOR
LMB763
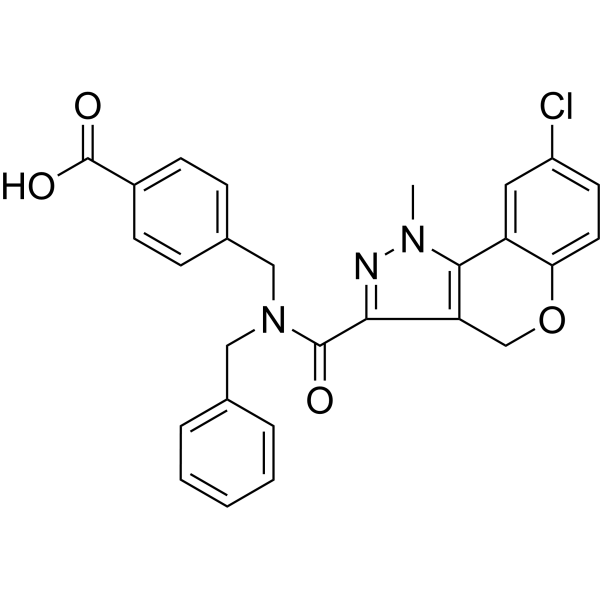
4-[[benzyl-(8-chloro-1-methyl-4H-chromeno[4,3-c]pyrazole-3-carbonyl)amino]methyl]benzoic acid
Nidufexor is a farnesoid X receptor (FXR) agonist.
| Molecular Weight |
487.93 |
|---|---|
| Formula |
C₂₇H₂₂ClN₃O₄ |
| CAS No. |
1773489-72-7 |
PHASE 2 Treatment of Liver and Biliary Tract Disorders,
Agents for Diabetic Nephropathy, NOVARTIS
1773489-72-7, LMB-763, UNII-CJ1PL0TE6J, CJ1PL0TE6J, BCP28929, EX-A1854
Nidufexor pound LMB-763 pound(c)
4-((N-benzyl-8-chloro-1-methyl-1,4-dihydrochromeno[4,3-c]pyrazole-3-carboxamido)methyl)benzoic acid

https://pubs.acs.org/doi/pdf/10.1021/acs.jmedchem.9b01621
1 (7.6 g, 89% yield) as a white solid. Melting point: 232.6 °C.
1 H NMR (400 MHz, DMSO): δ 12.93 (s, 1H), 7.96−7.85 (m, 2H), 7.71 (dd, J = 7.1, 2.5 Hz, 1H), 7.42−7.20 (m, 8H), 7.06 (dd, J = 8.7, 1.9 Hz, 1H), 5.45 (d, J = 3.9 Hz, 2H), 5.25 (d, J = 9.2 Hz, 2H), 4.58 (d, J = 12.1 Hz, 2H), 4.12 (d, J = 16.6 Hz, 3H).
13C NMR (101 MHz, DMSO-d6): δ 167.07, 162.21, 151.98, 142.65, 139.18, 132.20, 132.67, 129.70, 129.50, 129.50, 128.53, 128.53, 127.43, 127.43, 127.43, 127.43, 127.43, 125.53, 122.24, 119.0, 117.09, 116.64, 64.51, 50.68, 48.24. LC-MS m/z: 488.2/490.2 (M +H)+ ; chlorine pattern; method 3; RT = 1.41 min.
Elemental Analysis calcd for C27H22ClN3O4: C 66.46, H 4.54, N 8.61; found: C 66.43, H 4.56, N 8.62.
TRIS Salt Formation. Methanol (400 mL) was added to a mixture of 1 (4.0 g, 8.2 mmol) and 2-amino-2-hydroxymethylpropane-1,3-diol (TRIS, 1.0 g, 8.2 mmol). The mixture was heated to 70 °C for 0.5 h. After cooling to room temperature, the solvent was removed in vacuum. The residue was sonicated in dichloromethane (10 mL) and concentrated again. The resulting white solid was dried under vacuum overnight. The crude material was crystallized by slurring the solid residue in a 4:1 mixture of acetonitrile and methanol (5 mL). The mixture was stirred at room temperature for 24 h to give 4-((N-benzyl-8-chloro-1-methyl-1,4-dihydrochromeno- [4,3-c]pyrazole-3-carboxamido)methyl)benzoic acid TRIS salt as a white salt (3.7 g, 73% yield). Melting point: 195.6 °C. 1 H NMR (400 MHz, DMSO): δ 7.92−7.80 (m, 2H), 7.78−7.64 (m, 1H), 7.41− 7.19 (m, 8H), 7.13−7.00 (m, 1H), 5.44 (s, 2H), 5.25−5.14 (m, 2H), 4.61−4.48 (m, 2H), 4.18−4.03 (m, 3H), 3.39 (s, 7H). TRIS OH masked by water peak. LC-MS m/z: 488.0/490.0 (M+H)+ ; chlorine pattern, method 3. RT = 1.58 min. Elemental Analysis calc for C31H33ClN4O7: C 61.00, H 5.36, N 9.15; found: C 60.84, H 5.34, N 9.13.






https://pubs.acs.org/doi/suppl/10.1021/acs.jmedchem.9b01621/suppl_file/jm9b01621_si_001.pdf
Patent
WO 2015069666
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015069666&tab=PCTDESCRIPTION
///////NIDUFEXOR, LMB 763, Phase II, PHASE 2, Liver and Biliary Tract Disorders, Diabetic Nephropathy, NOVARTIS
CN1C(C2=CC(Cl)=CC=C2OC3)=C3C(C(N(CC4=CC=CC=C4)CC5=CC=C(C(O)=O)C=C5)=O)=N1
