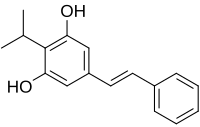
Benvitimod, Tapinarof
3,5-dihydroxy-4-isopropyl-trans-stilbene
Launched – 2019 CHINA, Psoriasis, Tianji Pharma
тапинароф [Russian] [INN]WBI-1001
DMVT-505
GSK-2894512
RVT-505
WB-1001
WBI-1001
84HW7D0V04 (UNII code)
Benvitimod is in phase III clinical trials, Dermavant Sciences for the treatment of atopic dermatitis and psoriasis.
The compound was co-developed by Welichem Biotech and Stiefel Laboratories (subsidiary of GSK). However, Shenzhen Celestial Pharmaceuticals acquired the developement rights in China, Taiwan, Macao and Hong Kong.
Benvitimod (also known as Tapinarof or 3,5-dihydroxy-4-isopropyl-trans-stilbene) is a bacterial stilbenoid produced in Photorhabdus bacterial symbionts of Heterorhabditis nematodes.It is a product of an alternative ketosynthase-directed stilbenoids biosynthesis pathway. It is derived from the condensation of two β-ketoacyl thioesters. It is produced by the Photorhabdus luminescens bacterial symbiont species of the entomopathogenic nematode, Heterorhabditis megidis.
Benvitimod (also known as tapinarof or 3,5-dihydroxy-4-isopropyl-trans-stilbene) is a bacterial stilbenoid produced in Photorhabdus bacterial symbionts of Heterorhabditis nematodes. It is a product of an alternative ketosynthase-directed stilbenoids biosynthesis pathway. It is derived from the condensation of two β-ketoacyl thioesters .[1] It is produced by the Photorhabdus luminescens bacterial symbiont species of the entomopathogenic nematode, Heterorhabditis megidis. Experiments with infected larvae of Galleria mellonella, the wax moth, support the hypothesis that the compound has antibiotic properties that help minimize competition from other microorganisms and prevents the putrefaction of the nematode-infected insect cadaver.[2]
Tapinarof is a non-steroidal anti-inflammatory drug originated by Welichem Biotech. Dermavant Sciences is developing the product outside China in phase III clinical trials for the treatment of plaque psoriasis. The company is also conducting phase II clinical trials for the treatment of atopic dermatitis. Phase II studies had also been conducted by Welichem Biotech and Stiefel (subsidiary of GlaxoSmithKline) for these indications.
Tapinarof was originated at Welichem Biotech, from which Tianji Pharma and Shenzen Celestial Pharmaceuticals obtained rights to the product in the Greater China region in 2005. In 2012, Welichem licensed development and commercialization rights in all other regions to Stiefel. In 2013, Welichem entered into an asset purchase agreement to regain Greater China rights to the product from Tianji Pharma and Celestial; however, this agreement was terminated in 2014. In 2018, Stiefel transferred its product license to Dermavant Sciences.
Entomopathogenic nematodesemerging from a wax moth cadaver
Medical research
Benvitimod is being studied in clinical trials for the treatment of plaque psoriasis.[3]
PATENTS

Patent
https://patents.google.com/patent/CN103992212A/en
phenalkenyl Maude (Benvitimod) is a new generation of anti-inflammatory drugs, are useful for treating a variety of major autoimmune diseases, such as psoriasis, eczema, hair and more concentrated colitis allergic diseases.Phenalkenyl Maud stilbene compound, comprising cis and trans isomers, the trans alkenyl benzene Maude has a strong physiological activity, stability and physical and chemical properties, and cis alkenyl benzene Modesto predominantly trans phenalkenyl Maud byproducts during synthesis, conventional methods such as benzene alkenyl Maude Wittig reaction of cis-isomer impurity is inevitable.
[0004] benzyl trans-alkenyl Maude as main impurities in the synthesis, whether a drug is detected, or monitored during the reaction, the synthesis and analysis methods established cis alkenyl benzene Maude has very important significance.Phenalkenyl Maud conventional synthetic methods the impurity content is very low, and the properties of the cis compound is extremely unstable, easily converted to trans-structure, the synthetic method according to the preceding, the cis compound difficult to separate. The synthesis method has not been reported before in the literature. Thus, to find a synthesis route of cis-alkenyl benzene Maude critical.
[0005] The synthesis of compounds of cis-stilbene, in the prior art, there have been many reports, however, the prior art method of synthesizing a reaction product of the cis starting materials and reagents difficult source, the catalyst used is expensive higher costs, operational difficulties, is not conducive to large-scale production, such as:
① Gaukroger K, John A.Hadfield.Novel syntheses of cis and trans isomers ofcombretastatin A-4 [J] .J.0rg.Chemj 2001, (66): 8135-8138, instead of styrene and substituted phenyl bromide boric acid as the raw material, the Suzuki coupling reaction is a palladium catalyst, to give the cis compound, the reaction follows the formula:
Yield and selectivity of the process the structure is good, but the reaction is difficult source of raw materials, catalyst more expensive, limiting the use of this method.
[0006] ② Felix N, Ngassaj Erick A, Lindsey, Brandon Ej Haines.The first Cu- and
amine-free Sonogashira-type cross-coupling in the C_6 -alkynylation of protected
2, -deoxyadenosine [J] .Tetrahedron Letters, 2009, (65): 4085-4091, with a substituted phenethyl m
Alkynyl easily catalyst Pd / CaC03, Fe2 (CO) 9, Pd (OAc) 2 and the like produce cis compound to catalytic reduction. The reaction follows the formula:
Advantage of this method is stereospecific reduction of alkynes in the catalyst, to overcome the phenomenon of cis-trans isomerization of the Wittig reaction, but the reaction requires at _78 ° C, is not conducive to the operation, and the reagent sources difficult, expensive than high cost increase is not conducive to mass production.
[0007] ③ Belluci G, Chiappe C, Moro G L0.Crown ether catalyzed stereospecificsynthesis of Z_and E-stilbenes by Wittig reaction in a solid-liquid two-phasessystem [J] .Tetrahedron Letters, 1996, (37): 4225-4228 using Pd (PPh3) 4 as catalyst, an organic zinc reagent with a halide compound of cis-coupling reaction formula as follows:
The advantage of this method is that selective, high yield to give cis; deficiency is difficult to handle, the catalyst is expensive.
[0008] ④ new Wang, Zhangxue Jing, Zhou Yue, Zouyong Shun, trans-3,4 ‘, 5-trihydroxy-stilbene China Pharmaceutical Synthesis, 2005, 14 (4);. 204-208, reported that the trans compound of formula was dissolved in DMSO solution at a concentration dubbed, ultraviolet irradiation was reacted at 365nm, converted into cis compounds, see the following reaction formula:
However, the concentration of the solution preparation method, the reaction time is more stringent requirements.

The synthesis of cis-alkenyl benzene Maude application embodiments Example 1 A synthesis of cis-alkenyl Maude benzene and benzene-cis-ene prepared Maude, the reaction was carried out according to the following scheme:
Specific preparation process steps performed in the following order:
(O methylation reaction
The 195.12g (Imol) of 3, 5-hydroxy-4-isopropyl benzoic acid, 414.57g (3mol) in DMF was added 5000ml anhydrous potassium carbonate, mixing, stirred at room temperature, then cooled in an ice-salt bath next, slowly added dropwise 425.85g (3mol) of iodomethane, warmed to room temperature after the addition was complete, the reaction 2h, after completion of the reaction was stirred with water, extracted with ethyl acetate, and concentrated to give 3,5-dimethoxy-4- isopropyl benzoate; yield 93%, purity of 99%.
[0033] (2) a reduction reaction
3000ml tetrahydrofuran and 240g (Imol) 3,5-dimethoxy-4-isopropyl benzoate, 151.40g (4mol) mixing at room temperature sodium borohydride was stirred and heated to reflux was slowly added dropwise 400ml methanol, reaction 4h, was added 3L of water was stirred, extracted with ethyl acetate, washed with water, the solvent was removed by rotary evaporation to give a white solid, to give 3,5-dimethoxy-4-isopropylbenzene methanol; 96% yield purity was 99%.
[0034] (3) the oxidation reaction
The 212g (ImoI) of 3,5-dimethoxy-4-isopropylbenzene methanol, DMSO 800ml and 500ml of acetic anhydride were mixed and stirred at rt After 2h, stirred with water, extracted with ethyl acetate, washed with water, dried , and concentrated to give 3,5-dimethoxy-4-isopropyl-benzaldehyde; 94% yield, 99% purity.
[0035] (4) a condensation reaction
The mixture was 209.18g (lmol) of 3,5-dimethoxy-4-isopropyl-benzoic awake and 136.15g (Imol) phenylacetic acid was added 5000ml of acetic anhydride, stirred to dissolve, sodium acetate was added 246.09g , heating to 135 ° C, the reaction after 6h, cooled to room temperature after adjusting the dilute acid 2 was added, extracted with ethyl acetate, the pH was concentrated, added saturated sodium bicarbonate solution adjusted to pH 7, stirred 2h, and extracted with dichloromethane , adding dilute aqueous hydrochloric acid pH 2, the yellow solid was filtered, to obtain 3,5-dimethoxy-4-isopropyl-stilbene acid; 96% yield, 80% purity.
[0036] (5) decarboxylation reaction
The 327g (Imol) of 3,5-dimethoxy-4-isopropyl-stilbene acid and 384g (6mol) of copper powder were added to 5000ml of quinoline, 180 ° C reaction 3h, cooled to room temperature ethyl acetate was added with stirring, filtered, and the filtrate was washed with dilute hydrochloric acid to the aqueous layer was colorless and the aqueous phase was extracted with ethyl acetate inverted, the organic layers were combined, washed with water and saturated brine until neutral, i.e., spin-dried to give 3,5 – dimethoxy-4-isopropyl-stilbene; 92% yield, 77% purity.
[0037] (6) Demethylation
The 282.32g (Imol) of 3,5-dimethoxy-4-isopropyl-stilbene 4000ml toluene was placed in an ice bath and stirring, was cooled to 0 ° C, and dissolved slowly added 605.9g (5mol after) in N, N- dimethylaniline, was added 666.7g (5mol) of anhydrous aluminum chloride. after stirring for 0.5h, warmed to room temperature, the reaction was heated to 100 ° C 2h, cooled to 60 ° C , hot toluene layer was separated, diluted hydrochloric acid was added to the aqueous phase with stirring to adjust the PH value of 2, extracted with ethyl acetate, washed with water, and concentrated to give the cis-alkenyl benzene Modesto; crude yield 95%, purity 74 %.After separation by column chromatography using 300-400 mesh silica gel, benzene-cis-ene was isolated Maude pure, 68% yield, 98.5% purity. The resulting cis-alkenyl benzene Maud NMR shown in Figure 1, NMR data are as follows:
1HNMR (CDCl3, 500 Hz, δ: ppm), 7.255 (m, 5H), 6.558 (d, 1H), 6.402 (d, 1H), 6.218 (s, 2H), 4.872 (s, 2H), 3.423 (m , 1H), 1.359 (q, 6H). Coupling constants / = 12.
[0038] trans-alkenyl benzene Maud NMR shown in Figure 2, the following NMR data:
1HNMR (CDCl3, 500 Hz, δ: ppm), 7.477 (d, 2H), 7.360 (t, 2H), 6.969 (q, 2H), 6.501 (s, 1H), 4.722 (s, 2H), 3.486 (m , 1H), 1.380 (t, 6H). Coupling constants / = 16.
[0039] HPLC conditions a cis alkenyl benzene Maude pure product: column was Nucleosil 5 C18; column temperature was 20 ° C; detection wavelength 318nm; mobile phase consisting of 50:50 by volume of acetonitrile and water; flow rate It was 0.6mL / min, injection volume of 5 μ L; cis phenalkenyl Maude 18.423min retention time of a peak in an amount of 96.39%, see Figure 3. Trans phenalkenyl Maude 17.630min retention time of a peak, the content was 99.8%, see Figure 4.After mixing the two, trans-alkenyl benzene Maude 17.664min retention time of the peak, cis-alkenyl benzene Maude 18.458min retention time of the peak, see Figure 5.
PATENT
https://patents.google.com/patent/CN103172497A/en

phenalkenyl Maude is a natural product, a metabolite as to be symbionts.Phenalkenyl Maud Escherichia coli, Staphylococcus aureus has a very significant inhibitory effect, in addition, there is a styrenic Maude suppression of inflammation and its reactive derivative with immunomodulating activity. Alkenyl benzene Modesto topical ointment as an active ingredient, as a class of drugs has been completed two clinical treatment of psoriasis and eczema, the results of ongoing clinical phase III clinical studies, it has been shown to be completed in both psoriasis and eczema clearly effect, together with a styrenic Maude is a non-hormonal natural small molecule compounds, can be prepared synthetically prepared, therefore, it exhibits good market prospect.
[0004] a styrenic Maude initial synthesis route is as follows:
[0005]
[0006] The reaction conditions for each step: 1) isopropanol, 80% sulfuric acid, 60 ° C, 65% .2) sodium borohydride, boron trifluoride, tetrahydrofuran, 0 ° C, 90% .3). of thionyl chloride, heated under reflux, 85% .4). triethyl phosphate, 120 ° C, 80% .5). benzaldehyde, sodium hydride, 85% .6) pyridine hydrochloride, 190 ° C, 60 %.
[0007] The chemical synthesis route, although ultimately obtained a styrenic Maude, but the overall yield is low, part of the reaction step is not suitable for industrial production, due to process conditions result in the synthesis of certain byproducts produced is difficult to remove impurities, difficult to achieve the quality standard APIs.
Preparation of 4-isopropyl-dimethoxy-benzoic acid [0011] 1,3,5_
[0012] 1000 l reactor 200 liters of 80% sulfuric acid formulation (V / V), the temperature was lowered to room temperature, put 80 kg 3,5_-dimethoxybenzoate ,, stirring gradually warmed to 60 ° C, in was added dropwise within 25 kg of isopropanol I hour, the reaction was complete after 5 hours, 500 liters of hot water, filtered, the filter cake was washed with a small amount of hot water I th, crushed cake was removed and dried. The dried powder was recrystallized from toluene, the product was filtered to give 78 kg `, yield 86%. Preparation 2,3,5_ dimethoxy-4-isopropylbenzene methanol
[0013] 1000 l reactor was added 50 kg 3,5_ _4_ isopropyl dimethoxy benzoic acid, 24 kg of potassium borohydride, 400 l of THF, at room temperature was slowly added dropwise 65 kg BF3.Et2O was stirred 12 hours, the reaction was complete, pure water was added dropwise to destroy excess BF3, filtered, concentrated to dryness, methanol – water to give an off-white recrystallized 40.3 kg, yield 90.1%.
[0014] Preparation of 3,3,5-_ ■ methoxy _4- isopropyl group gas section
[0015] 1000 l autoclave, 100 kg of 3,5-dimethoxy-4-isopropylbenzene methanol, 220 l of DMF, 0 ° C and added dropwise with stirring and 50 l of thionyl chloride, 24 hours after the reaction was complete, 300 liters of water and 300 liters of ethyl acetate, the aqueous phase was stirred layered discharged, and then washed with 200 liters of water was added 3 times, until complete removal of DMF, was added concentrated crystallized from petroleum ether to give 98 kg of white solid was filtered and dried a yield of 91%.
Preparation of methyl-dimethoxy-4-isopropylbenzene of diethyl [0016] 4,3,5_
[0017] 500 l autoclave, 98 kg 3,5_ _4_ isopropyl dimethoxy benzyl chloride and 120 l of triethyl phosphite, the reaction at 120 ° C 5h, fear distilled off under reduced pressure, the collection 145-155 ° C / 4mmHg fear minutes, cured at room temperature to give a colorless light solid was 118 kg, yield 81.6%.
, 3- [0018] 5, E-1 _ ■ methoxy-2-isopropyl-5- (2-phenylethyl lean-yl) – benzene
[0019] 500 l autoclave, 33 kg 3,5_-dimethoxy-4-isopropylbenzene acid diethyl ester, 10.8 kg of benzaldehyde, and 120 l of tetrahydrofuran, at 40 ° C, and nitrogen with stirring, was added dropwise a solution of 11.8 kg potassium tert-butoxide in 50 liters of tetrahydrofuran, the temperature dropping control not to exceed 50 ° C. after the dropwise addition stirring was continued for I h, the reaction was complete, 150 liters of ethyl acetate and extracted , washed twice with 150 liters of water, 100 l I washed with brine, and the organic phase was dried and concentrated, methanol – water (I: D as a white crystalline solid 25.3 kg, yield 91%.
[0020] 6> 1, 3 ~ _ ■ Light-2-isopropyl-5- (2-phenylethyl lean-yl) – benzene (I), (De Dae dilute benzene)
[0021] 100 l autoclave, 10 kg 1,3_-dimethoxy-2-isopropyl-5- (2-styryl) benzene _ pyridine hydrochloride and 25 kg nitrogen atmosphere was heated to 180 -190 ° C, stirred for 3 hours after the reaction was completed, 20 l HCl (2N) cooling to 100 ° C, and 20 liters of ethyl acetate the product was extracted, dried and concentrated to give the product 7.3 kg, 83% yield.
[0022] The method for purifying:
[0023] 100 l added to the reaction vessel 15.5 kg of crude product and 39 liters of toluene, heated to the solid all dissolved completely, filtered hot and left to crystallize, after crystallization, filtration, the crystals with cold toluene 10 washed liter at 60 ° C, protected from light vacuo dried for 24 hours, to obtain 14 kg of white needle crystals, yield 90%.
CLIP
https://www.eosmedchem.com/article/237.html
Design new synthesis of Route of Benvitimod
Benvitimod 79338-84-4 intermediate: 1999-10-5
Benvitimod 79338-84-4 intermediate: 2150-37-0
Benvitimod 79338-84-4 intermediate: 344396-17-4
Benvitimod 79338-84-4 intermediate: 344396-18-5
Benvitimod 79338-84-4 intermediate: 344396-19-6
Benvitimod 79338-84-4 intermediate: 1080-32-6
Benvitimod 79338-84-4 intermediate: 678986-73-7
Benvitimod 79338-84-4 intermediate: 55703-81-6
Benvitimod 79338-84-4 intermediate: 1190122-19-0
Benvitimod 79338-84-4 intermediate: 443982-76-1
Benvitimod 79338-84-4 intermediate: 100-52-72.ROS-Benvitimod
(1)
(2)
Name: Benvitimod
CAS#: 79338-84-4
Chemical Formula: C17H18O2
Exact Mass: 254.1307
Molecular Weight: 254.329
Elemental Analysis: C, 80.28; H, 7.13; O, 12.58
References
- ^ Joyce SA; Brachmann AO; Glazer I; Lango L; Schwär G; Clarke DJ; Bode HB (2008). “Bacterial biosynthesis of a multipotent stilbene”. Angew Chem Int Ed Engl. 47 (10): 1942–5. doi:10.1002/anie.200705148. PMID 18236486.
- ^ Hu, K; Webster, JM (2000). “Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus–Heterorhabditis infected Galleria mellonella larvae”. FEMS Microbiology Letters. 189 (2): 219–23. doi:10.1111/j.1574-6968.2000.tb09234.x. PMID 10930742.
- ^ “New Topical for Mild to Moderate Psoriasis in the Works”. Medscape. March 5, 2017.
- https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fanie.201814016&file=anie201814016-sup-0001-misc_information.pdf
///Benvitimod, Tapinarof, WBI-1001, тапинароф , تابيناروف , 他匹那罗 , Welichem Biotech, Stiefel Laboratories, Shenzhen Celestial Pharmaceuticals,CHINA 2019 , Psoriasis, Tianji Pharma, Dermavant Sciences, PHASE 3










