Image may be NSFW.
Clik here to view.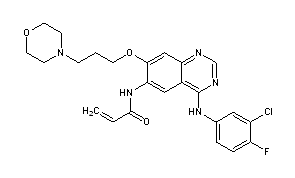
Image may be NSFW.
Clik here to view.
CANERTINIB
Clik here to view.
Canertinib (CI-1033) is an experimental drug candidate for the treatment of cancer. It is an irreversible tyrosine-kinase inhibitor with activity against EGFR (IC50 0.8 nM), HER-2 (IC50 19 nM) and ErbB-4 (IC50 7 nM).[1][2] By 2015, Pfizer had discontinued development of the drug.[3]
Canertinib has been reported as a substrate for OATP1B3. Interaction of canertinib with OATP1B3 may alter its hepatic disposition and can lead to transporter mediated drug-drug interactions.[4] Also, canertinib is not an inhibitor of OATP-1B1 or OATP-1B3 transporter.[5]
SYN
J Med Chem 2000,43(7),1380
| EP 1131304; US 6344455; WO 0031048 |
4-Chloro-7-fluoro-6-nitroquinazoline (I) was condensed with 3-chloro-4-fluoroaniline (II) to afford the 4-anilino quinazoline (III). Displacement of the activated fluorine of (III) with the potassium alkoxide of morpholinopropanol (IV) gave the morpholinopropyl ether (V). Subsequent reduction of the nitro group of (V), either using iron dust and acetic acid or catalytic hydrogenation over Raney-Ni, furnished aminoquinazoline (VI). This was finally condensed with acrylic acid (VII), via activation as the mixed anhydride with isobutyl chloroformate or using EDC as the coupling reagent, to provide the title acrylamide.
Image may be NSFW.
Clik here to view.
PATENT
https://patents.google.com/patent/CN103242244A/en
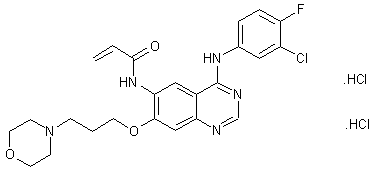
canertinib (Canertinib, I), chemical name 4- (3-chloro-4-fluoroanilino) -7- [3- (4_-morpholinyl) propoxy] -6-propylene quinazoline amide group, and by the US Pfizer Warner Lambert developed jointly an irreversible epidermal growth factor receptor (pan-ErbB) selective inhibitor, which is capable of binding to the cell surface of all members of the ErbB family adenosine triphosphate binding site, thereby inhibiting the activation of these receptors and their downstream mitogenic signal transduction pathways. Clinical studies show that the product has good resistance, can be effective in treating metastatic breast cancer, ovarian cancer, cervical cancer and other tumors, and can be combined with a variety of antineoplastic agents exhibit a synergistic effect.
[0004]
[0005] China Patent No. CN1160338C, CN1438994A and No. No. CN1745073A reported the preparation of canertinib: A nucleus 4- [(3-chloro-4-fluorophenyl) amino] -6-nitro 7-fluoro-quinazoline (VIII) as a starting material, under basic conditions with 3- (4-morpholinyl) -1-propanol 7-position substitution reaction occurs to give 4- [(3-chloro – 4-fluorophenyl) amino] -6-nitro-7- [3- (4-morpholinyl) -1-propoxy] quinazoline (IX); intermediate (IX) through the 6-position nitro reduction, to give the corresponding amino compound (X); amino compound (X) to give canertinib acylation reaction (I) with acrylic acid or acryloyl chloride occurs.
[0006] In addition, “Qilu Pharmaceutical Affairs” 30, 2011, Vol. 10, page 559, and “China Industrial Medicine” 2010 Volume 41, No. 6, pp. 404 also reported an improved method of the above-prepared and studied method from 7-fluoro-quinazolin-3-one (V) via nitration, chloro and condensation reaction of the preparation of intermediate (VIII) is.
[0007]
[0008] This shows that the current Kanai prepared for Nepal is mainly the 4-position through an intermediate (VII), respectively, a functional transformation of the 6-position and 7-position achieved. Since the intermediate (VII) a fluorine-containing compounds, materials are not readily available, many steps, and many steps are required to be isolated and purified by column chromatography, which is not required for industrialization.
Example a:
[0023] at room temperature, to a three-necked flask was added diisopropyl azodicarboxylate (3mL, 15mmol) and tetrahydrofuran 5mL, dropwise addition of triphenylphosphine (4.0g, 15mmol) in tetrahydrofuran 25mL solution at room temperature, kept at room temperature for 2 hours. Under nitrogen, 3- (4-morpholinyl) -1_-propanol (0.49g, 3.4mmol) in 5mL of tetrahydrofuran was added dropwise to the reaction system after the dropwise addition is complete, 6-amino – 7-hydroxy-3,4-dihydro-quinazolin-4-one (II) (0.53g,
3.0mmol), stirred at room temperature for 4 hours. Solution of 3- (4-morpholinyl) -1-propanol (0.38g, 2.6mmol) in 5mL of tetrahydrofuran was continued at room temperature for 2 hours, the end of the reaction was monitored TLC. Recovery of the solvent by distillation under reduced pressure, the residue was treated with dilute hydrochloric acid, pH = 5-6, extracted with ethyl acetate, the organic phase was washed with saturated sodium carbonate adjusted pH = 10-11. The aqueous phase was freeze-dried in vacuo to give an off-white solid 6-amino-7- [3- (4-morpholinyl) propoxy] _3,4- dihydroquinazolin-4-one (111) 0.80g yield 87.7%.
[0024] Example II:
[0025] to a three-neck flask was added 6-amino-7- [3- (4_ morpholino) propoxy] quinazolin-dihydro _3,4_ one _4_
(III) (0.76g, 2.5mmol), triethylamine (0.25g, 2.5mmol) and dichloromethane 20mL, warmed to 40-45 ° C, stirred until homogeneous dissolution system. Dropped below 10 ° C, was slowly added dropwise acryloyl chloride (0.25g, 2.8mmol) in dichloromethane IOmL solution dropwise at room temperature after continued for 6 h, TLC detection reaction was completed. The reaction solution was respectively 10% sodium bicarbonate solution and water, dried over anhydrous sodium sulfate. Recovery of the solvent under reduced pressure, the residue was recrystallized from ethyl acetate to give a white solid 7- [3- (4-morpholinyl) propoxy] -6-acrylamido-3,4-dihydro-quinazoline – 4-one (IV) 0.81g, 90.5% yield.
[0026] Example III:
Under [0027] nitrogen, to a three-necked flask was added 7- [3- (4_-morpholinyl) propoxy] -6-acrylamido-_3,4- dihydroquinazolin-4-one (IV ) (3.58g, IOmmol), benzotriazol-1-yloxytris (dimethylamino) phosphonium iron hexafluorophosphate (BOP) (6.63g, 15mmol) and acetonitrile 100mL. Under stirring, a solution of 1,8-diazabicyclo [5.4.0] ^ a-7-ene (DBU) (2.28g, 15mmol), dropwise, at room temperature for 12 hours. Warmed to 60 ° C, the reaction was continued for 12 hours. The solvent was removed by distillation under reduced pressure, ethyl acetate was added to dissolve IOOmL, washed with 2M sodium hydroxide and 20mL. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in tetrahydrofuran IOOmL, 4-chloro-3-fluoroaniline (1.89g, 13mmol) and sodium hydride (0.32g, 13mmol), was heated to 50 ° C, reaction was stirred for 5 hours, the end of the reaction was monitored TLC. Quenched with saturated brine the reaction, the organic phase was separated, dried, evaporated under reduced pressure to recover the solvent to give an off-white solid. Recrystallized from ethanol to give an off-white solid canertinib (I) 4.05g, yield 83.5%.
[0028] Example IV:
Under [0029] nitrogen, to a three-necked flask was added 7- [3- (4_-morpholinyl) propoxy] -6-acrylamido-3,4-dihydro-quinazolin-4-one (IV ) (3.58g, IOmmol), benzotriazol-1-yloxytris (dimethylamino) phosphonium iron hexafluorophosphate (BOP) (6.63g, 15mmol) and acetonitrile lOOmL. Under stirring, dropwise power port I, 5- diazabicyclo [4.3.0] – non-5-ene (DBN) (1.86g, 15mmol), dropwise, at room temperature for 12 hours. Warmed to 60 ° C, the reaction was continued for 12 hours. The solvent was removed by distillation under reduced pressure, ethyl acetate was added to dissolve IOOmL, washed with 2M sodium hydroxide and 20mL. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in tetrahydrofuran IOOmL, 4-chloro-3-fluoroaniline (1.89g, 13mmol) and sodium hydride (0.32g, 13mmol), was heated to 50 ° C, reaction was stirred for 5 hours, the end of the reaction was monitored TLC. Quenched with saturated brine the reaction, the organic phase was separated, dried, evaporated under reduced pressure to recover the solvent to give an off-white solid. Recrystallized from ethanol to give an off-white solid canertinib (I) 3.85g, yield 79.4%. ·
[0030] Example Five:
Under [0031] nitrogen, to a three-necked flask was added 7- [3- (4_-morpholinyl) propoxy] -6-acrylamido-3,4-dihydro-quinazolin-4-one (IV ) (3.58g, IOmmol), benzotriazol-1-yloxytris (dimethylamino) phosphonium hexafluorophosphate gun (BOP) (6.63g, 15mmol), 4_-chloro-3-fluoroaniline ( 1.89g, 13mmol) and N, N- dimethylformamide lOOmL. Under stirring, a solution of I, 8- diazabicyclo [5.4.0] – ^ a _7_ ene (DBU) (2.28g, 15mmol), dropwise, at room temperature for 12 hours. Warmed to 60 ° C, the reaction was continued for 12 hours. The solvent was removed by distillation under reduced pressure, ethyl acetate was added to dissolve IOOmL, washed with 2M sodium hydroxide and 20mL. The organic phase was separated, dried and concentrated under reduced pressure. The residue was recrystallized from ethanol to give an off-white solid canertinib (1) 2.32g, yield 47.8%.
Image may be NSFW.
Clik here to view.
References
GW; Loo, JA; Greis, KD; Chan, OH; Reyner, EL; Lipka, E; Showalter, HD; et al. (2000). “Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4-(phenylamino)quinazoline- and 4-(phenylamino)pyrido3,2-dpyrimidine-6-acrylamides bearing additional solubilizing functions”. Journal of Medicinal Chemistry. 43 (7): 1380–97. doi:10.1021/jm990482t. PMID 10753475.
- ^ CI-1033 (Canertinib), Selleck Chemicals
- ^ http://adisinsight.springer.com/drugs/800012072
- ^ Khurana V, Minocha M, Pal D, Mitra AK (March 2014). “Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors”. Drug Metabol Drug Interact. 29 (3): 1–11. doi:10.1515/dmdi-2013-0062. PMC 4407685. PMID 24643910.
- ^ Khurana V, Minocha M, Pal D, Mitra AK (May 2014). “Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors”. Drug Metabol Drug Interact. 29 (4): 1–11. doi:10.1515/dmdi-2014-0014. PMC 4407688. PMID 24807167.
| Image may be NSFW. Clik here to view. 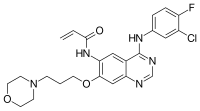 |
|
| Names | |
|---|---|
| IUPAC name
N-{4-[(3-Chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}prop-2-enamide
|
|
| Other names
CI-1033; PD-183805
|
|
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
|
PubChem CID
|
|
| UNII |
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C24H25ClFN5O3 | |
| Molar mass | 485.94 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
/////////////CANERTINIB
C=CC(=O)NC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4



