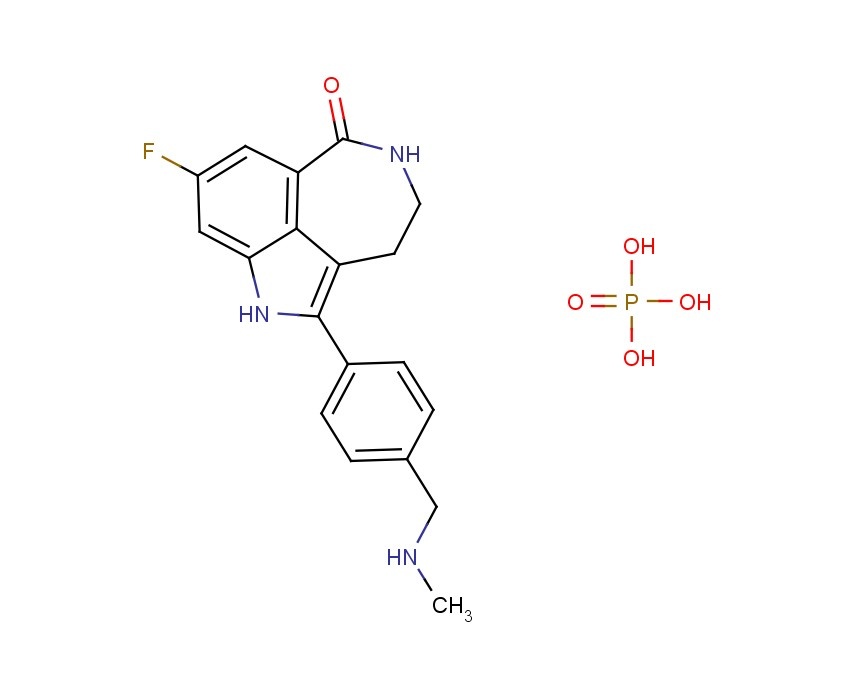
AG014699,
AG014699, the phosphate salt of AG14447, which has improved aqueous solubility, has been selected for clinical trial.AG014699 is a tricyclic indole poly(ADP-Ribose) polymerase (PARP) inhibitor with potential antineoplastic activity.
M.Wt: 421.3593
Formula: C19H21FN3O5P
CAS No: 459868-92-9

Rucaparib, PF-01367338283173-50-2 cas 6H-Pyrrolo[4,3,2-ef][2]benzazepin-6-one, 8-fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-6H- Azepino[5,4,3-cd]indol-6-one, 8-fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl] -8-Fluoro-2-[4-[(methylamino)methyl]phenyl]-1,3,4,5- tetrahydro-6H-azepino[5,4,3-cd]indol-6-one;8-Fluoro-2-(4-methylaminomethyl-phenyl)-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one8-Fluoro-2-(4-methylaminomethyl-phenyI)-l,3,4,5-tetrahydro-azepino[5,4,3- cd]indol-6-one
- MW..C19 H18 F N3 O
- cas of csa salt—–1327258-57-0
- 773059-19-1 (hydrochloride)
773059-22-6 (L-tartrate)
773059-23-7 (acetate) - 459868-92-9 PHOSPHATE
- AG-014699
AG-14699
CO-338
PF-01367338
AG-014447 (free base)
AG-14447 (free base) - Agouron (Originator)
Pfizer (Originator) - Clovis Oncology
- WO 2014052550, WO 2014037313, WO 2000042040WO 2004087713WO 2005012305
![]()
Rucaparib (AG 014699) is a PARP inhibitor being investigated as a potential anti-cancer agent.
Rucaparib inhibits “the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture.”[1]
It can be taken orally in tablet form.[2]
It has undergone phase I clinical trials for patients with advanced solid tumours.[3] It is in phase II clinical trials for metastatic breastand ovarian cancer with known BRCA1 or BRCA2 mutation.[4][2]
It is thought that 20% of women with ovarian cancer who are not BRCA positive might also benefit from PARP inhibitors. Clinical trials are beginning (as of April, 2014)
As of November 2012 four clinical trials of rucaparib were recruiting patients.[5]
Inhibition of poly(ADP ribose) polymerase, or PARP, is an exciting new mechanism for the treatment of cancer.(1) The PARP enzyme is responsible for repair of damaged DNA in both normal and tumor cells, and inhibition of this repair mechanism is expected to make the cell more likely to undergo apoptosis. Preclinical work has shown that PARP inhibitors coadministered with a standard chemotherapuetic agent are more effective than the standard treatment aloneRucaparib is a NAD+ ADP-ribosyltransferase inhibitor in phase II clinical development at Cancer Research UK for the treatment of patients with advanced ovarian cancer and in patients with locally advanced or metastatic breast cancer. Clovis Oncology is conducting early clinical evaluation of rucaparib for the treatment of triple negative breast cancer or ER/PR +, HER2 negative with known BRCA1/2 mutations p2 and for the treatment of gBRCA mutation breast cancer.. Pfizer discontinued development of rucaparibin 2011.In 2011, the compound was licensed to Clovis Oncology by Pfizer for the treatment of cancer. In 2012, orphan drug designation was assigned in the U.S. and the E.U. for the treatment of ovarian cancer.
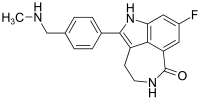
The compound 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H-azepino[5,4,3- cd]indol-6-one represented by formula
is a small molecule inhibitor of poly(ADP-ribose) polymerase (PARP). 8-Fluoro-2-{4- [(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one and salts thereof, is disclosed in U.S. Patent No. 6,495,541 and PCT Application No. PCT/IB2004/000915, International Publication No. WO 2004/087713, the disclosures of which are incorporated herein by reference in their entireties. U.S. Provisional Patent Applications No. 60/612,459 and 60/679,296, entitled “Polymorphic Forms of the Phosphate Salt of 8-Fluoro-2-{4-[(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H- azepino[5,4,3-cd]indol-6-one,” the disclosures of which are incorporated herein by reference in their entireties, describe novel polymorphic forms of the phosphate salt of 8-fluoro-2-{4- [(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one, and methods for their preparation. U.S. Provisional Patent Applications No. 60/612,458; and 60/683,006, entitled “Therapeutic Combinations Comprising Poly(ADP-Ribose) Polymerases Inhibitor,” the disclosures of which are incorporated herein by reference in its entirety, describe pharmaceutical combinations of 8-fluoro-2-{4- [(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one.
………………………………………
PATENT
http://www.google.com/patents/WO2000042040A1?cl=enExample IIII:8-Fluoro-2-(4-methylaminomethyl-phenyI)-l,3,4,5-tetrahydro-azepino[5,4,3- cd]indol-6-one
4-(8-fluoro-6-oxo-3,4,5,6-tetrahydro-lH-azepino[5,4,3-cd]indol-2-yl)- benzaldehyde (100 mg, 0.32 mmol; prepared in a manner similar to that described for compound 12 for 2-bromo-8-fluoro-l,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one and 4-formylphenylboronic acid) was reacted with methylamine (1.62 mmol) as described for Compound PPP to yield 8-fluoro-2-(4-methylaminomethyl-phenyl)- l,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one, 32 mg (31%) as a yellow solid: m.p. 1543-155 °C; Η NMR (300 MHz, d6-DMSO) 2.28 (s, 3H), 3.04 (m, 2H), 3.40 (m, 2H), 3.69 (s, 2H), 7.32 (dd, 7= 9.0, 2.4 Hz, IH), 7.44 (m, 3H), 7.57 (d, 7= 8.1 Hz, 2H), 8.25 (br t, IH), 11.67 (br s, IH). HRMS (MALDI MH+) Calcd for C19H18N3OF: 324,1512. Found: 325.1524. Anal. (C19H18N3OF03 H2O) C, H, N.
……………………………..
PAPER
http://pubs.acs.org/doi/full/10.1021/op200238p Novel PARP inhibitor 1 is a promising new candidate for treatment of breast and ovarian cancer. A modified synthetic route to 1 has been developed and demonstrated on 7 kg scale. In order to scale up the synthesis to multikilogram scale, several synthetic challenges needed to be overcome. The key issues included significant thermal hazards present in a Leimgruber–Batcho indole synthesis, a low-yielding side-chain installation, a nonrobust Suzuki coupling and hydrogen cyanide generation during a reductive amination. In addition to these issues, changing from intravenous to oral delivery required a new salt form and therefore a new crystallization procedure. This contribution describes development work to solve these issues and scaling up of the new process in the pilot plant.
Novel PARP inhibitor 1 is a promising new candidate for treatment of breast and ovarian cancer. A modified synthetic route to 1 has been developed and demonstrated on 7 kg scale. In order to scale up the synthesis to multikilogram scale, several synthetic challenges needed to be overcome. The key issues included significant thermal hazards present in a Leimgruber–Batcho indole synthesis, a low-yielding side-chain installation, a nonrobust Suzuki coupling and hydrogen cyanide generation during a reductive amination. In addition to these issues, changing from intravenous to oral delivery required a new salt form and therefore a new crystallization procedure. This contribution describes development work to solve these issues and scaling up of the new process in the pilot plant.
The compound 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H-azepino[5,4,3- cd]indol-6-one represented by formula
is a small molecule inhibitor of poly(ADP-ribose) polymerase (PARP). 8-Fluoro-2-{4- [(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one and salts thereof, is disclosed in U.S. Patent No. 6,495,541 and PCT Application No. PCT/IB2004/000915, International Publication No. WO 2004/087713, the disclosures of which are incorporated herein by reference in their entireties.
U.S. Provisional Patent Applications No. 60/612,459 and 60/679,296, entitled “Polymorphic Forms of the Phosphate Salt of 8-Fluoro-2-{4-[(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H- azepino[5,4,3-cd]indol-6-one,” the disclosures of which are incorporated herein by reference in their entireties, describe novel polymorphic forms of the phosphate salt of 8-fluoro-2-{4- [(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one, and methods for their preparation. U.S. Provisional Patent Applications No. 60/612,458; and 60/683,006, entitled “Therapeutic Combinations Comprising Poly(ADP-Ribose) Polymerases Inhibitor,” the disclosures of which are incorporated herein by reference in its entirety, describe pharmaceutical combinations of 8-fluoro-2-{4- [(methylamino)methyl]phenyl}-1 ,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one.


Example 13. Synthesis of 8-Fluoro-2-(4-methylaminomethyl-phenyl)-1,3.4.5-tetrahvdro-azepinor5.4.3- ccflindol-6-one (15) i
Lactam 14 (14.42 g, 0.038 mol) was dissolved in hydrobromic acid in acetic acid (30%-32%, 140 ml). The reaction solution was stirred for 46 hours at room temperature in a 500ml flask that was connected to an ethanolamine scrubber system. HPLC analysis indicated the completion of the reaction. Ice (30 g) was added to the reaction solution followed by addition of aqueous NaOH (327 ml, 10 M, 3.27 mol) while the temperature was maintained between 25 0C and 35 0C. When addition of NaOH was complete, the pH was 10. The resulting solid was collected by filtration, washed with water (2 x 50 ml). The filter cake was then suspended in water (125 ml) and stirred for 2 hours. The solid was collected by filtration, washed with water (2 x 25 ml) and dried to afford 10.76 g of product (88% yield). 1H NMR (300 MHz, DMSO-d6) δ 2.577(s, 3H), 3.053(m, 2H), 3.406(m, 2H), 4.159(s, 2H), 7.36(dd, 1 H, J= 2.4 Hz and J= 9.3 Hz), 7.44(dd, 1 H, J= 2.4 Hz and J= 11.1 Hz), 7.63(d, 2H, J=8.1 Hz), 7.70(d, 2H, J= 8.1 Hz), 8.265(t, 1H, J= 5.7 Hz), 11.77(s, 1 H). Exact mass calculated for C19H19FN3O: 324.1512. Found: 324.1497.
 |
|
| Names | |
|---|---|
| IUPAC name
8-Fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one
|
|
| Other names
AG014699
|
|
| Identifiers | |
283173-50-2  |
|
| ChemSpider | 8107584 |
| Jmol-3D images | Image |
| KEGG | D10079  |
| PubChem | 9931954 |
| Properties | |
| C19H18FN3O | |
| Molar mass | 323.37 g·mol−1 |
References
- http://www.qub.ac.uk/schools/SchoolofPharmacy/Filestore/Filetoupload,121186,en.pdf
- “Cancer Research launches new drug trial”. 11 Jan 2011.
- “First in human phase I trial of the PARP inhibitor AG-014699 with temozolomide (TMZ) in patients (pts) with advanced solid tumors”.
- http://science.cancerresearchuk.org/research/loc/newcastle/newcastle_univ/plummerr/plummerrfr/plummerrfrp2/?version=1 URL no longer relevant
- Rucaparib trials
Clovis Oncology receives Breakthrough Therapy designation for rucaparib for treatment of advanced ovarian cancer in patients with BRCA-mutated tumours
7 April 2015 • Author: Victoria White
Clovis Oncology has announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation for the Company’s investigational agent rucaparib as monotherapy treatment of advanced ovarian cancer in patients who have received at least two lines of prior platinum-containing therapy, with BRCA-mutated tumours, inclusive of both germline BRCA (gBRCA) and somatic BRCA (sBRCA) mutations.

2525 28th Street
Suite 100
Boulder, CO 80301
Tel: 303.625.5000
Fax: 303.245.0360

are a biopharmaceutical company focused on acquiring, developing and commercializing cancer treatments in the United States, Europe and other international markets. Our development programs are targeted at specific subsets of cancer, combining personalized medicine with companion diagnostics to direct therapeutics to those patients most likely to benefit from them.
We have three product candidates in clinical development: rociletinib (CO-1686), which is in Phase II development for the treatment of non-small cell lung cancer; rucaparib, which is in Phase II and Phase III clinical trials for the treatment of ovarian cancer; and lucitanib, which is in Phase II clinical trials for the treatment of breast and lung cancers. We have received Breakthrough Therapy designation from the FDA for rociletinib and rucaparib. We maintain global rights for rociletinib and rucaparib, and U.S. and Japanese rights to lucitanib.
Boulder, Colorado
-
Boulder, Colorado – Wikipedia, the free encyclopedia
en.wikipedia.org/wiki/Boulder,_ColoradoLocation in Boulder County and the State of Colorado. Coordinates: … ZIP codes,80301-80310, 80314, 80321-80323, 80328, 80329. Area code(s), Both 303 …
![]()
![]()



///////
Filed under: Breakthrough Therapy Designation, Uncategorized Tagged: AG 014699, AG014699, Boulder, Breakthrough Therapy Designation, Colorado, metastatic breast cancer, methylamino, ovarian cancer, PARP, Provisional Patent Applications, rucaparib









