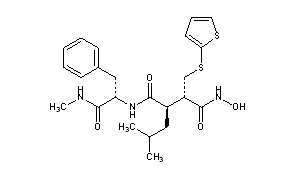

| Formula |
C23H31N3O4S2
|
|---|---|
| cas |
130370-60-4
|
| Mol weight |
477.6399
|
Batimastat (INN/USAN, codenamed BB-94) is an anticancer drug that belongs to the family of drugs called angiogenesis inhibitors. It acts as a matrix metalloproteinase inhibitor (MMPI) by mimicking natural MMPI peptides.
Batimastat was the first MMPI that went into clinical trials. First results of a Phase I trial appeared in 1994. The drug reached Phase III but was never marketed; mainly because it couldn’t be administered orally (as opposed to the newer and chemically similar MMPI marimastat), and injection into the peritoneum caused peritonitis.[1]
SYN
U.S. Patent 5,453,438
U.S. Patent 5,240,958
U.S. Patent 5,530,161

SYN
US 5240958; US 5310763; WO 9005719
The treatment of D-leucine (I) with NaNO2, H2SO4 and NaBr gives 2(R)-bromo-5-methylpentanoic acid (II), which is esterified with isobutene and H2SO4 to the corresponding tert-butyl ester (III). The condensation of (III) with dibenzyl malonate (IV) by means of potassium tert-butoxide in DMF yields the malonyl derivative (V), which is treated with trifluoroacetic acid to hydrolyze the tert-butyl ester, and without isolation is condensed with L-phenylalanine methyl amide (VI) by means of hydroxybenzotriazole (HOBT) and dicyclohexylcarbodiimide (DCC), affording 4-benzyloxy-3-(benzyloxycarbonyl)-2(R)-isobutylsuccinyl-L-phenylalanine methylamide (VII). The elimination of the benzyl groups of (VII) by hydrogenolysis over Pd/C in ethanol gives the dicarboxylic acid (VIII), which by partial decarboxylation and reaction with aqueous formaldehyde and piperidine yields 4-hydroxy-2(R)-isobutyl-3-methylenesuccinyl-L-phenylalanine methylamide (IX). The addition of thiophene-2-thiol (X) to the double bond of (IX) affords 4-hydroxy-2(R)-isobutyl-3(S)-(2-thienylsulfanylmethyl)succinyl-L-phenylalanine methylamide (XI), which is finally treated with hydroxylamine and hydroxybenzotriazole in dichloromethane/DMF.

SPEC

HPLC

References
- ^ Rothenberg, M. L.; Nelson, A. R.; Hande, K. R. (1999). “New Drugs on the Horizon: Matrix Metalloproteinase Inhibitors”. Stem Cells. 17 (4): 237–240. doi:10.1002/stem.170237. PMID 10437989.
 |
|
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration |
Injection into pleural space or abdomen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.222.897  |
| Chemical and physical data | |
| Formula | C23H31N3O4S2 |
| Molar mass | 477.64 g/mol g·mol−1 |
| 3D model (JSmol) | |
//////////Batimastat, BB-94, バチマスタット ,
[H][C@@](CC1=CC=CC=C1)(NC(=O)[C@]([H])(CC(C)C)[C@]([H])(CSC1=CC=CS1)C(=O)NO)C(=O)NC