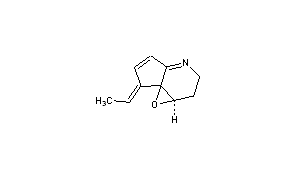
Abikoviromycin
- Molecular FormulaC10H11NO
- Average mass161.200 Da
CAS Registry Number: 31774-33-1
CAS Name: 7-Ethylidene-1a,2,3,7-tetrahydrocyclopent[b]oxireno[c]pyridine
(1aR,7E,7aS)-7-Ethylidene-1a,2,3,7-tetrahydrocyclopenta[b]oxireno[c]pyridine [ACD/IUPAC Name]
abikoviromycin
Cyclopent(b)oxireno(c)pyridine, 7-ethylidene-1a,2,3,7-tetrahydro-, (1aR,7E,7aS)-
Cyclopent[b]oxireno[c]pyridine, 7-ethylidene-1a,2,3,7-tetrahydro-, (1aR,7E,7aS)-
Additional Names: 4,4a-epoxy-5-ethylidene-2,3,4,4a-tetrahydro-5H-1-pyridine; abicoviromycin; latumcidin
Molecular Formula: C10H11NO
Molecular Weight: 161.20
Percent Composition: C 74.51%, H 6.88%, N 8.69%, O 9.93%
Literature References: Antiviral antibiotic produced by Streptomyces abikoensis and Streptomyces rubescens. Chromatographic isoln from broth cultures: Umezawa et al., Jpn. Med. J. 4, 331 (1951); C.A. 46, 7167 (1952); Umezawa, JP 54 6200 (1954 to Nippon). Identity with latumcidin: Sakagami et al., J. Antibiot. 11A, 231 (1958). Structure: Gurevich et al., Tetrahedron Lett. 1968,2209. Stereochemistry: Kono et al., J. Antibiot. 23, 572 (1970); Gurevich et al., Khim. Prir. Soedin. 7, 104 (1971), C.A. 75, 5752e (1971). Crystal and molecular structure of the selenate: Y. Kono et al., Acta Crystallogr. B27, 2341 (1971). In vitro antiviral activity: V. M. Roikhel, N. A. Zeitlenok, Antibiotiki 14, 969 (1969), C.A. 72, 19394q (1969).
Properties: Highly unstable and polymerizes promptly on isolation even at -50°; however, it can be handled in dilute solutions and in the form of its salts. uv max (neutral ethanol or 0.1N KOH): 218, 244, 289 nm (log e 3.83, 3.99, 3.94); (0.1N HCl) 236, 341 nm (log e 3.99, 4.05).
Absorption maximum: uv max (neutral ethanol or 0.1N KOH): 218, 244, 289 nm (log e 3.83, 3.99, 3.94); (0.1N HCl) 236, 341 nm (log e 3.99, 4.05)
Isolation of abikoviromycin and dihydroabikoviromycin as inhibitors of polyketide synthase involved in melanin biosynthesis by Colletotrichum lagenarium
Journal of Antibiotics (2003), 56, (9), 801-804.
Journal of Antibiotics (2003), 56, (9), 801-804.
purified by normal-phase HPLC (column: Senshu-Pak Aquasil SS-752N, 10×250mm, Senshu Kagaku; mobile phase: isocratic elution of nhexane: 2-propanol: H2O: triethylamine, 70:30:1:0.02; flow rate: 5ml/minutes; retention time: 9.0 minutes) to obtain 1 (6.3mg). 1:
FAB-MS (NBA matrix) m/z 162 (M+H)+; [α]20D+67.5° (c 0.025, 0.1N NaOH) [lit. [α]21D +148.9° (c 1, 0.1N NaOH)]9);
1H NMR (500MHz, CDCl3) δ 7.43 (1H, d, J=6.5Hz, 7-H), 6.53 (1H, d, J=6.5Hz, 6- H), 5.50 (1H, q, J=7.0Hz, 8-H), 3.92 (1H, s, 4-H), 3.81 (1H, dd, J=5.5, 15Hz, 2-Ha), 3.69 (1H, dt, J=5.5, 15Hz, 2-Hb), 2.19 (1H, m, 3-Ha), 1.90 (3H, d, J=7.0Hz, 9-H), 1.62 (1H, m, 3-Hb);
13C NMR (125MHz, CDCl3) δ 172.1 (C-7a), 142.3 (C-7), 136.5 (C-5), 132.8 (C-6), 119.5 (C-8), 59.5 (C-4), 54.5 (C-4a), 44.5 (C-2), 21.8 (C-3),14.0 (C-9).
////////////Abikoviromycin
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE![]() amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////



 amcrasto@gmail.com
amcrasto@gmail.com