Image may be NSFW.
Clik here to view.
Vandetanib; 443913-73-3; Zactima; ZD6474; Caprelsa; ZD 6474;
Vandetanib (INN, trade name Caprelsa) is an anti-cancer drug that is used for the treatment of certain tumours of the thyroid gland. It acts as a kinase inhibitor of a number of cell receptors, mainly the vascular endothelial growth factor receptor (VEGFR), theepidermal growth factor receptor (EGFR), and the RET-tyrosine kinase.[1][2] The drug was developed by AstraZeneca.
Image may be NSFW.
Clik here to view.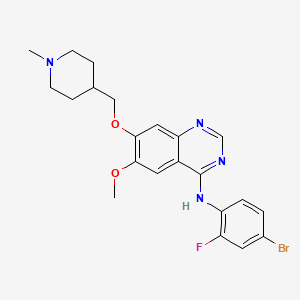
Approvals and indications
Vandetanib was the first drug to be approved by FDA (April 2011) for treatment of late-stage (metastatic) medullary thyroid cancer in adult patients who are ineligible for surgery.[3] Vandetanib was first initially marketed without a trade name,[4] and is being marketed under the trade name Caprelsa since August 2011.[5]
2011 年 4 月 6 by the FDA-approved surgical resection can not be used for locally advanced or metastatic medullary thyroid cancer (medullary thyroid cancer, MTC) of the drug. Vandetanib is vascular endothelial growth factor receptors (vascular endothelial growth factor receptor, VEGFR) and epidermal growth factor receptor (epidermal growth factor receptor, EGFR) antagonists, tyrosine kinase inhibitors (tyrosine kinase inhibitor). Produced by AstraZeneca.
The synthetic route is as follows:Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Pharmacokinetics
Vandetanib is well absorbed from the gut, reaches peak blood plasma concentrations 4 to 10 hours after application, and has a half-life of 120 hours days on average, per Phase I pharmacokinetic studies. It has to be taken for about three months to achieve a steady-state concentration. In the blood, it is almost completely (90–96%) bound to plasma proteins such as albumin. It is metabolised to N-desmethylvandetanib via CYP3A4 and to vandetanib-N-oxide via FMO1 and 3. Both of these are active metabolites. Vandetanib is excreted via the faeces (44%) and the urine (25%) in form of the unchanged drug and the metabolites.[2][9][10]
Image may be NSFW.
Clik here to view.
Metabolites of vandetanib (top left): N-desmethylvandetanib (bottom left, via CYP3A4), vandetanib-N-oxide (bottom right, via FMO1 andFMO3), both pharmacologically active, and a minor amount of aglucuronide.[10]
Clinical trials
Non-small cell lung cancer
The drug underwent clinical trials as a potential targeted treatment for non-small-cell lung cancer. There have been some promising results from a phase III trial withdocetaxel.[11] There have also been ambivalent results when used with pemetrexed.[12] Another trial with docetaxel was recruiting in July 2009.[13]
AstraZeneca withdrew EU regulatory submissions for vandetanib (under the proposed trade name Zactima) in October 2009 after trials showed no benefit when the drug was administered alongside chemotherapy.[14]
References
- “Definition of vandetanib”. NCI Drug Dictionary. National Cancer Institute.
- “Vandetanib Monograph”. Drugs.com. Retrieved 29 August 2012.
- “FDA approves new treatment for rare form of thyroid cancer”. Retrieved 7 April 2011.
- Jump up^ “FDA approves orphan drug vandetanib for advanced medullary thyroid cancer” (Press release). AstraZeneca. Retrieved 2011-08-17.
- “AstraZeneca announces trade name CAPRELSA® for vandetanib” (Press release). AstraZeneca. Retrieved 2011-08-17.
- Khurana V, Minocha M, Pal D, Mitra AK (March 2014). “Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors.”. Drug Metabol Drug Interact.0 (0): 1–11. doi:10.1515/dmdi-2013-0062. PMID 24643910.
- Haberfeld, H, ed. (2012). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- Jump up^ Khurana V, Minocha M, Pal D, Mitra AK (May 2014). “Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors.”. Drug Metabol Drug Interact. 0 (0): 1–11.doi:10.1515/dmdi-2014-0014. PMID 24807167.
- Martin, P.; Oliver, S.; Kennedy, S. J.; Partridge, E.; Hutchison, M.; Clarke, D.; Giles, P. (2012). “Pharmacokinetics of Vandetanib: Three Phase I Studies in Healthy Subjects”.Clinical Therapeutics 34 (1): 221–237. doi:10.1016/j.clinthera.2011.11.011.PMID 22206795.
- ^ Jump up to:a b “Clinical Pharmacology Review: Vandetanib” (PDF). US Food and Drug Administration, Center for Drug Evaluation and Research. 20 August 2010. Retrieved29 August 2012.
- “Vandetanib Shows Clinical Benefit When Combined With Docetaxel For Lung Cancer”. ScienceDaily. 3 June 2009.
- Jump up^ “IASLC: Vandetanib Fails to Improve NSCLC Outcomes with Pemetrexed”. Medpage today. 5 Aug 2009.
- Clinical trial number NCT00687297 for “Study of Vandetanib Combined With Chemotherapy to Treat Advanced Non-small Cell Lung Cancer” at ClinicalTrials.gov
- “Zactima”. European Medicines Agency.
External links[
| Image may be NSFW. Clik here to view. 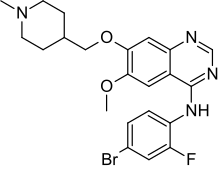 |
|
| Systematic (IUPAC) name | |
|---|---|
|
N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)methoxy]quinazolin-4-amine
|
|
| Clinical data | |
| Trade names | Caprelsa |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611037 |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration |
Oral |
| Pharmacokinetic data | |
| Protein binding | 90–96% |
| Metabolism | CYP3A4, FMO1, FMO3 |
| Biological half-life | 120 hours (mean) |
| Excretion | 44% faeces, 25% urine |
| Identifiers | |
| CAS Registry Number | 443913-73-3 Image may be NSFW. Clik here to view.  |
| ATC code | L01XE12 |
| PubChem | CID: 3081361 |
| IUPHAR/BPS | 5717 |
| DrugBank | DB08764 Image may be NSFW. Clik here to view.  |
| ChemSpider | 2338979 Image may be NSFW. Clik here to view.  |
| UNII | YO460OQ37K Image may be NSFW. Clik here to view.  |
| ChEBI | CHEBI:49960 Image may be NSFW. Clik here to view.  |
| ChEMBL | CHEMBL24828 Image may be NSFW. Clik here to view.  |
| Synonyms | ZD6474 |
| Chemical data | |
| Formula | C22H24BrFN4O2 |
| Molecular mass | 475.354 g/mol |
//////
Filed under: Uncategorized Tagged: ASTRAZENECA, medullary thyroid cancer, VANDETANIB Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.

