Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Alatrofloxacin Mesylate
| Chemical Names: | Alatrofloxacin mesylate; UNII-2IXX802851; 146961-77-5; Alatrofloxacin mesylate [USAN]; 157605-25-9; 2IXX802851 |
|---|---|
| Molecular Formula: | C27H29F3N6O8S |
| Molecular Weight: | 654.618 g/mol |
| CAS No. | 146961-76-4 (Alatrofloxacin ); 157605-25-9 (Alatrofloxacin Mesylate); |
| Chemical Name | (1α, 5α, 6α)-L-alanyl-N-[3-[6-carboxy-8-(2,4-difluorophenyl)-3-fluoro-5,8-dihydro-5-oxo-1,8-naphthyridine-2-yl]-3-azabicyclo[3.1.0]hex-6-yl]-L-alaninamide, monomethanesulfonate |
Research Code:CP-116517-27; CP-116517, Trade Name:Trovan I.V.® MOA:Quinolone antibiotic Indication:Life- or limb-threatening infections caused by susceptible strains Status:Withdrawn Company:Pfizer (Originator)
Alatrofloxacin (Trovan IV) is a fluoroquinolone antibiotic developed by Pfizer, delivered as a mesylate salt.[1]
Trovafloxacin and alatrofloxacin were both withdrawn from the U.S. market in 2001
Alatrofloxacin mesylate was first approved by the U.S. Food and Drug Administration (FDA) on Dec 18, 1997. It was developed and marketed as Trovan I.V. ® by Pfizer in the US.
Alatrofloxacin mesylate is a fluoronaphthyridone related to the fluoroquinolones with in vitro activity against a wide range of gram-negative and gram-positive aerobic and anaerobic microorganisms. The bactericidal action of alatrofloxacin results from inhibition of DNA gyrase and topoisomerase IV. Trovan I.V.® is indicated for the treatment of patients initiating therapy in in-patient health care facilities (i.e., hospitals and long term nursing care facilities) with serious, life- or limb-threatening infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Trovan I.V.® is available as injection solution for intravenous use, containing 7.86 mg/ml of Alatrofloxacin mesylate. The recommended starting dose is 200 mg or 300 mg administered intravenously.
Alatrofloxacin mesylate was withdrawn from the U.S. market in 2001.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Alatrofloxacin mesilate
-
- Synonyms:CP 116517, CP 116517-27
- ATC:J01MA
- Use:antibiotic, prodrug of trovafloxacin
- Chemical name:l-Alanyl-N-[(1α,5α,6α)-3-[6-carboxy-8-(2,4-difluorophenyl)-3-fluoro-5,8-dihydro-5-oxo-1,8-naphthyridin-2-yl]-3-azabicyclo[3.1.0]hex-6-yl]-l-alaninamide monomethanesulfonate
- Formula:C26H25F3N6O5 • CH4O3S
- MW:654.62 g/mol
- CAS-RN:146961-77-5
Derivatives
base
- Formula:C26H25F3N6O5
- MW:558.52 g/mol
- CAS-RN:146961-76-4
Substance Classes
Synthesis Path
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.
Substances Referenced in Synthesis Path
| CAS-RN | Formula | Chemical Name | CAS Index Name |
|---|---|---|---|
| 27317-69-7 | C11H20N2O5 | N–tert-butoxycarbonyl-l-alanyl-l-alanine | L-Alanine, N-[(1,1-dimethylethoxy)carbonyl]-L-alanyl- |
| 186772-86-1 | C33H37F3N6O7 | N-[(1,1-dimethylethoxy)carbonyl]-l-alanyl-N-[(1α,5α,6α)-3-[8-(2,4-difluorophenyl)-6-(ethoxycarbonyl)-3-fluoro-5,8-dihydro-5-oxo-1,8-naphthyridin-2-yl]-3-azabicyclo[3.1.0]hex-6-yl]-l-alaninamide | L-Alaninamide, N-[(1,1-dimethylethoxy)carbonyl]-L-alanyl-N-[(1α,5α,6α)-3-[8-(2,4-difluorophenyl)-6-(ethoxycarbonyl)-3-fluoro-5,8-dihydro-5-oxo-1,8-naphthyridin-2-yl]-3-azabicyclo[3.1.0]hex-6-yl]- |
| 171176-56-0 | C22H19F3N4O3 | ethyl (1α,5α,6α)-7-(6-amino-3-azabicyclo[3.1.0]hex-3-yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylate | 1,8-Naphthyridine-3-carboxylic acid, 7-(6-amino-3-azabicyclo[3.1.0]hex-3-yl)-1-(2,4-difluorophenyl)-6-fluoro-1,4-dihydro-4-oxo-, ethyl ester, (1α,5α,6α)- |
| 134575-66-9 | C27H27F3N4O5 | ethyl (1α,5α,6α)-1-(2,4-difluorophenyl)-7-[6-[[(1,1-dimethylethoxy)carbonyl]amino]-3-azabicyclo[3.1.0]hex-3-yl]-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylate | 1,8-Naphthyridine-3-carboxylic acid, 1-(2,4-difluorophenyl)-7-[6-[[(1,1-dimethylethoxy)carbonyl]amino]-3-azabicyclo[3.1.0]hex-3-yl]-6-fluoro-1,4-dihydro-4-oxo-, ethyl ester, (1α,5α,6α)- |
| 75-75-2 | CH4O3S | methanesulfonic acid | Methanesulfonic acid |
Trade Names
| Country | Trade Name | Vendor | Annotation |
|---|---|---|---|
| D | TROVAN | Pfizer | wfm |
| F | Turvel | Pfizer | wfm |
| GB | Turvel | Pfizer | wfm |
| I | Turvel | Pfizer | wfm |
| USA | Trovan | Pfizer | wfm |
(wfm = withdrawn from market)
Formulations
- vial 200 mg/40 ml, 300 mg/60 ml (5 mg/ml) (as mesilate)
References
-
- US 5 164 402 (Pfizer; 17.11.1992; appl. 4.2.1991; WO-prior. 16.8.1989).
- US 5 229 396 (Pfizer; 20.7.1993; appl. 24.7.1992).
- WO 9 700 268 (Pfizer; appl. 27.3.1996; USA-prior. 15.6.1995).
- US 5 763 454 (Pfizer; 9.6.1998; appl. 21.5.1997; WO-prior. 6.6.1995).
-
polymorphs:
- US 6 080 756 (Pfizer; 27.6.2000; appl. 30.1.1998; WO-prior. 5.7.1996).
References
“Center for Drug Evaluation and Research – Application Number: 020759/020760 – Chemistry Review(s)” (PDF). Food and Drug Administration. Retrieved 29 August 2014.
| Image may be NSFW. Clik here to view. 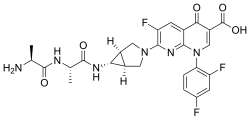 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a605016 |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 76% (trovafloxacin) |
| Metabolism | Quickly hydrolyzed to trovafloxacin |
| Elimination half-life | 9 to 12 hours (trovafloxacin) |
| Excretion | Fecal and renal(trovafloxacin) |
| Identifiers | |
| CAS Number |
|
| ChemSpider |
|
| UNII | |
| ChEMBL |
|
| Chemical and physical data | |
| Formula | C26H25F3N6O5 |
| Molar mass | 558.509 g/mol |
| 3D model (JSmol) | |
/////////////////
